
|
시장보고서
상품코드
1766169
신경 도관, 신경 랩 및 신경 이식 수복 제품 시장 기회, 성장 촉진요인, 산업 동향 분석, 예측(2025-2034년)Nerve Conduit, Nerve Wrap, and Nerve Graft Repair Product Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
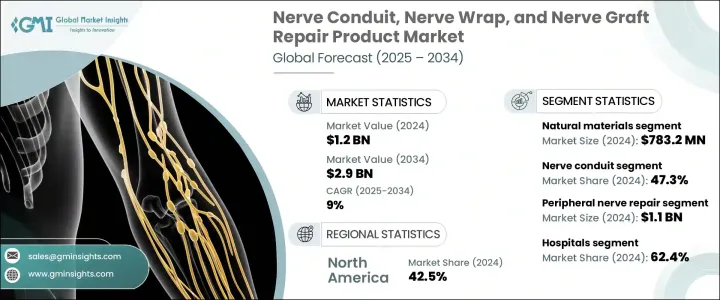
세계의 신경 도관, 신경 랩 및 신경 이식 수복 제품 시장은 2024년에 12억 달러로 평가되었고, 2034년에는 29억 달러에 이르며, CAGR은 9%를 나타낼 것으로 전망됩니다.
이 시장을 견인하는 것은 말초 신경 손상 증가, 생체 재료의 진보, 신경을 온존하는 솔루션에 대한 기호의 향상, 신경 수복에 사용되는 의료기기에 대한 규제상의 지원입니다. 노화와 같은 요인으로 인해 뇌와 척수 이외에서 발생하는 말초 신경 손상이 증가하고 있습니다.
교통사고, 노동재해, 스포츠 관련 사고, 관통 외상의 빈도가 증가하고 있기 때문에 신경도관, 랩, 이식편을 포함한 수술적 개입에 대한 수요가 현저하게 높아지고 있습니다. 조각과 같은 신경 복구 제품은 기능을 회복하고 신경 재생을 촉진하는 데 필수적인 해결책을 제공합니다. 교통 사고는 말초 신경 손상의 주요 원인입니다. 신경경로에 심각한 외상을 일으키는 경우가 많기 때문에 공장이나 건설 현장 등 리스크가 높은 환경에서의 노동재해도, 특히 육체 노동자의 신경 손상의 주된 원인이 되고 있습니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 12억 달러 |
| 예측 금액 | 29억 달러 |
| CAGR | 9% |
신경 도관 부문은 2024년에 47.3%의 최대 점유율을 차지했습니다. 도관은 그 사용의 용이성과 소-중 정도의 신경의 틈을 수복하는 능력으로부터, 임상 현장에서 지지되고 있습니다. 이러한 기구는 더 복잡하고 종종 공여자 부위의 합병증으로 이어지는 자가 이식을 대체하는 침습이 적고 긴장이 없는 대안을 제공합니다. 신경 도관은 신경의 틈에서 축삭을 유도하는 채널로 작용하여 적절한 신경 재생을 보장합니다. 특히 이식편에서는 너무 작은 신경의 틈새에 도관을 채용하는 케이스가 늘고 있어 시장 확대의 큰 원동력이 되고 있습니다.
천연 재료 부문은 2024년 7억 8,320만 달러를 기록해 생체 시스템과의 탁월한 적합성과 조직 통합을 강화할 수 있는 능력을 갖추었습니다. 이 물질은 신체의 자연적인 구조를 충실하게 모방하고 합성 대체품보다 효과적인 치유를 촉진하기 때문에 최적의 신경 재생을 촉진하는 데 중요한 역할을합니다. 콜라겐, 탈세포화 세포외 매트릭스(ECM), 가공 인간 이식편과 같은 천연 재료는 축삭 성장에 적합한 미세 환경을 생성하는 능력이 있기 때문에 선호됩니다.
2024년 미국의 신경 도관, 신경 랩 및 신경 이식 수복 제품 시장 규모는 4억 6,110만 달러로 평가되었는데, 외상 치료, 형성·재건 수술, 정형외과 수술, 교통 사고 등에 의한 신경 손상의 다발이 원동력이 되고 있습니다. FDA 인가의 신경이식편, 도관, 랩을 입수할 수 있는 것과, 신경 수복에 대한 유리한 상환 정책이, 전국의 병원이나 외래 진료소에서의 이러한 제품의 채용을 뒷받침하고 있습니다.
신경 도관, 신경 랩 및 신경 이식 수복 제품 시장 주요 기업은 Axogen, Axolotl Biologix, BioCircuit Technologies, Checkpoint Surgical, Collagen Matrix, Cook Group, Integra LifeSciences, KeriMedical, Medovent, Newrotex, Orthocell, Polyganics, Stryker Corporation, Synovis, 동양 방 등이 있습니다. 신경 도관, 신경 랩 및 신경 이식 수복 제품의 각 분야의 기업은 시장에서의 지위를 강화하기 위해, 제품 라인 업의 확충과, 말초 신경 손상 환자의 진화하는 요구에 응하는 혁신적 솔루션의 개발에 주력하고 있습니다. 기업은 조직 통합과 신경 재생을 강화하는 생체 재료에 중점을 두고 신경 복구 제품의 기능과 성능을 향상시키기 위한 연구 개발에 투자하고 있습니다.
목차
제1장 조사 방법과 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 공급자의 상황
- 밸류체인에 영향을 주는 요인
- 업계에 미치는 영향요인
- 성장 촉진요인
- 말초 신경 손상의 발생률의 상승
- 생체 재료의 진보
- 신경 온존의 대체 요법에 대한 임상적 취향 증가
- 규제 당국의 승인
- 업계의 잠재적 위험 및 과제
- 높은 제품 비용
- 장기적인 결과에 관한 임상적 증거의 부족
- 시장 기회
- 차세대 바이오엔지니어링 이식의 성장
- 암 절제 후 치과 수술 증가
- 성장 촉진요인
- 성장 가능성 분석
- 특허 분석
- 규제 상황
- 북미
- 유럽
- 아시아태평양
- 라틴아메리카
- 중동 및 아프리카
- 기술과 혁신의 상황
- 현재의 기술 동향
- 신흥기술
- 가격 분석
- 제품 유형별
- 지역별
- 향후 시장 동향
- 갭 분석
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 서론
- 기업의 시장 점유율 분석
- 기업 매트릭스 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 전략 대시보드
- 주요 발전
- 합병과 인수
- 파트너십 및 협업
- 신제품 발매
- 확장 계획
제5장 시장 추정·예측 : 제품 유형별(2021-2034년)
- 주요 동향
- 신경 도관
- 신경 랩
- 신경 그래프트
제6장 시장 추정·예측 : 재료별(2021-2034년)
- 주요 동향
- 천연 재료
- 합성 재료
제7장 시장 추정·예측 : 용도별(2021-2034년)
- 주요 동향
- 말초 신경 복구
- 치과 적용
- 중추 신경 복구
제8장 시장 추정·예측 : 최종 용도별(2021-2034년)
- 주요 동향
- 병원
- 외래수술센터(ASC)
- 학술연구기관
제9장 시장 추정·예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 프로파일
- Axogen
- Axolotl Biologix
- BioCircuit Technologies
- Checkpoint Surgical
- Collagen Matrix
- Cook Group
- Integra LifeSciences
- KeriMedical
- Medovent
- Newrotex
- Orthocell
- Polyganics
- Stryker Corporation
- Synovis
- Toyobo
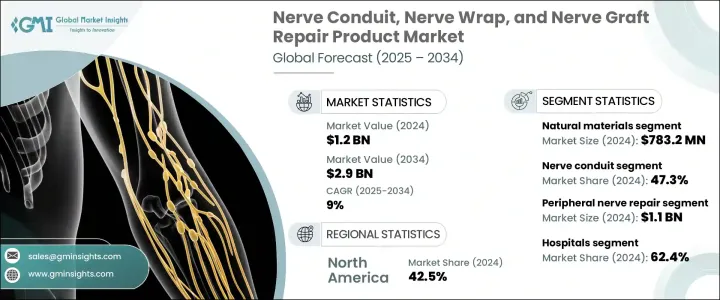
The Global Nerve Conduit, Nerve Wrap, and Nerve Graft Repair Product Market was valued at USD 1.2 billion in 2024 and is estimated to grow at a CAGR of 9% to reach USD 2.9 billion by 2034. This market is driven by a growing number of peripheral nerve injuries, advances in biomaterials, an increasing preference for nerve-sparing solutions, and regulatory support for medical devices used in nerve repair. Peripheral nerve injuries, which occur outside of the brain and spinal cord, are on the rise due to factors such as accidents, trauma, surgeries, and an aging population. These injuries commonly affect the upper limbs and often lead to loss of motor or sensory function, pain, and long-term psychosocial impacts.
The growing frequency of road traffic accidents, workplace injuries, sports-related accidents, and penetrating trauma has significantly amplified the demand for surgical interventions involving nerve conduits, wraps, and grafts. These injuries often result in extensive damage to peripheral nerves, particularly in the limbs, where direct suturing becomes challenging due to the size or location of the nerve gap. In such cases, nerve repair products like conduits, wraps, and grafts offer an essential solution for restoring functionality and promoting nerve regeneration. Road traffic accidents have become a major contributor to peripheral nerve injuries, as the force of impact often leads to severe trauma in critical nerve pathways. Similarly, workplace injuries, including those in high-risk environments such as factories or construction sites, continue to be a leading cause of nerve damage, especially among manual laborers.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.2 Billion |
| Forecast Value | $2.9 Billion |
| CAGR | 9% |
The nerve conduit segment held the largest share of 47.3% in 2024. Conduits are favored in clinical settings for their ease of use and ability to repair small to moderate nerve gaps. These devices provide a less invasive, tension-free alternative to autografts, which are more complex and often lead to complications at the donor site. Nerve conduits serve as channels that guide axons through nerve gaps, ensuring proper nerve regeneration. The growing adoption of conduits, especially for nerve gaps that are too small for grafts, has significantly driven market expansion.
The natural materials segment generated USD 783.2 million in 2024, driven by their superior compatibility with biological systems and their ability to enhance tissue integration. These materials play a critical role in facilitating optimal nerve regeneration, as they closely mimic the body's natural structure, promoting more effective healing than synthetic alternatives. Natural materials like collagen, decellularized extracellular matrices (ECM), and processed human allografts are preferred for their ability to create a conducive microenvironment for axonal growth.
U.S. Nerve Conduit, Nerve Wrap, and Nerve Graft Repair Product Market was valued at USD 461.1 million in 2024, driven by the high incidence of nerve injuries resulting from trauma care, plastic and reconstructive surgeries, orthopedic procedures, and road traffic accidents. The availability of FDA-approved nerve grafts, conduits, and wraps, coupled with favorable reimbursement policies for nerve repair, has helped drive the adoption of these products in hospitals and outpatient clinics across the country.
Leading companies in the Nerve Conduit, Nerve Wrap, and Nerve Graft Repair Product Market include Axogen, Axolotl Biologix, BioCircuit Technologies, Checkpoint Surgical, Collagen Matrix, Cook Group, Integra LifeSciences, KeriMedical, Medovent, Newrotex, Orthocell, Polyganics, Stryker Corporation, Synovis, and Toyobo. To strengthen their market position, companies in the nerve conduit, nerve wrap, and nerve graft repair sectors are focusing on expanding their product offerings and developing innovative solutions that cater to the evolving needs of patients with peripheral nerve injuries. Many companies are investing in research and development to improve the functionality and performance of nerve repair products, with an emphasis on biomaterials that enhance tissue integration and nerve regeneration. Additionally, strategic partnerships with hospitals and healthcare providers, along with collaborations with academic institutions, are helping companies advance their technological capabilities and clinical knowledge.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Technology
- 2.2.3 Treatment
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising incidence of peripheral nerve injuries
- 3.2.1.2 Advancements in biomaterials
- 3.2.1.3 Growing clinical preference for nerve-sparing alternatives
- 3.2.1.4 Supportive regulatory approvals
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of product
- 3.2.2.2 Lack of clinical evidence for long term outcomes
- 3.2.3 Market opportunities
- 3.2.3.1 Growth in next-generation bioengineered grafts
- 3.2.3.2 Rise in post-cancer resection and dental surgery
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Patent analysis
- 3.5 Regulatory landscape
- 3.5.1 North America
- 3.5.2 Europe
- 3.5.3 Asia Pacific
- 3.5.4 Latin America
- 3.5.5 Middle East & Africa
- 3.6 Technology and innovation landscape
- 3.6.1 Current technological trends
- 3.6.2 Emerging technologies
- 3.7 Pricing analysis
- 3.7.1 By Product type
- 3.7.2 By Region
- 3.8 Future market trends
- 3.9 Gap analysis
- 3.10 Porter's analysis
- 3.11 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategy dashboard
- 4.7 Key developments
- 4.7.1 Mergers & acquisitions
- 4.7.2 Partnerships & collaborations
- 4.7.3 New product launches
- 4.7.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Nerve conduit
- 5.3 Nerve wrap
- 5.4 Nerve grafts
Chapter 6 Market Estimates and Forecast, By Material, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Natural materials
- 6.3 Synthetic materials
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Peripheral nerve repair
- 7.3 Dental applications
- 7.4 Central nerve repair
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Ambulatory surgical centers
- 8.4 Academic research institutions
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Italy
- 9.3.5 Spain
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Axogen
- 10.2 Axolotl Biologix
- 10.3 BioCircuit Technologies
- 10.4 Checkpoint Surgical
- 10.5 Collagen Matrix
- 10.6 Cook Group
- 10.7 Integra LifeSciences
- 10.8 KeriMedical
- 10.9 Medovent
- 10.10 Newrotex
- 10.11 Orthocell
- 10.12 Polyganics
- 10.13 Stryker Corporation
- 10.14 Synovis
- 10.15 Toyobo



















