
|
시장보고서
상품코드
1766173
OTC 결핍증 치료 시장 기회, 성장 촉진요인, 산업 동향 분석, 예측(2025-2034년)Ornithine Transcarbamylase Deficiency Treatment Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
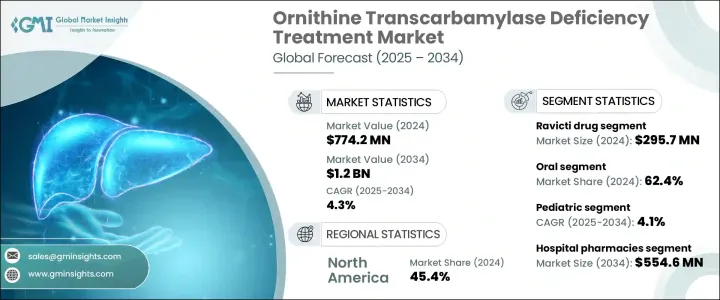
세계의 OTC 결핍증 치료 시장은 2024년에는 7억 7,420만 달러로 평가되었고, 2034년에는 12억 달러에 이르며, CAGR은 4.3%를 나타낼 것으로 전망됩니다.
우레아 사이클 장애(UCD)의 이환율의 상승, 정밀의료와 유전자 치료에 있어서의 지속적인 기술 혁신이 시장 확대의 원동력이 되고 있습니다.

유전자 편집 기술, 효소 보충 치료, 간을 표적으로 한 저분자 화합물 등의 차세대 치료법의 개발은 연구와 임상시험에 대한 다액의 투자에 의해 지원되고 있습니다. 프레임 워크는 시장을 더욱 발전시키고 있습니다. 의료 업계의이 분야는 우레아 사이클이 손상되어 혈중 암모니아 농도가 증가하고 지속적인 관리가 필요한 생명을 위협하는 경우가 많을 때 드문 유전 질환을 다루는 치료제에 중점을 둡니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 7억 7,420만 달러 |
| 예측 금액 | 12억 달러 |
| CAGR | 4.3% |
라빅티 부문은 2024년에 2억 9,570만 달러를 창출했습니다. 이 부문은 특히 어린이와 노인에게 사용하기 쉽고 내약성이 향상되어 환자에게 높은 지원을 받고 있습니다. 이 요법은 여러 지역에서 규제 당국의 승인을 얻고 있으며, 다양한 의료 시스템에서의 이용 편의성과 섭취가 진행되고 있습니다.
경구투여부문은 2024년에 62.4%의 점유율을 차지했습니다. 경구투여가 선호되는 것은 그 편리성과, 지속적인 케어가 필요한 병태의 관리에 불가결한 빈번한 통원에의 의존을 경감하는 능력에 기인합니다. 진보는 경구 약물의 치료 효과를 향상시키고 정맥 내 투여와 비교할 수 있습니다.
미국의 OTC 결핍증 치료 시장은 2024년 시장 규모가 3억 1,560만 달러였습니다. Orphan Drug Act와 같은 지원 이니셔티브는 희귀 질병을 대상으로 하는 치료법의 개발과 신속한 승인을 뒷받침하고 있습니다.
세계의 OTC 결핍증 치료 시장을 형성하는 주요 기업은 Ultragenyx Pharmaceutical, Abbott Laboratories, Bausch Health Companies, Arcturus Therapeutics, Nutricia(Danone Group), Mead Johnson(Reckitt Benckiser) Therapeutics, OrphanPacific, Nestle, Amgen 등이 있습니다. 계약에 대한 투자, 희귀의약품의 지정에 의한 규제상의 우위성 확보 등이 포함됩니다. 또 많은 기업은 치료의 어드히어런스를 높이기 위해서 환자 지원 프로그램을 강화해, 안정된 공급 체인을 확보하기 위해서 제조 능력을 구축하고 있습니다.
목차
제1장 조사 방법과 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 업계에 미치는 영향요인
- 성장 촉진요인
- OTC 결핍증의 조기 발견에 대한 의식 향상
- 생명을 위협하는 질환에 대한 충족되지 않은 의료 요구 증가
- 공적 및 민간 보험 회사에 의한 강력한 보험과 상환
- 업계의 잠재적 위험 및 과제
- 승인된 치료법의 입수가 제한
- 치료비가 높아
- 업계의 잠재적 위험 및 과제
- 신생아 스크리닝의 확대에 의한 진단율의 향상
- 전문 약국과 희귀질환 인프라의 이용 가능성의 확대
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 북미
- 유럽
- 아시아태평양
- 향후 시장 동향
- 파이프라인 분석
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 서론
- 기업의 시장 점유율 분석
- 기업 매트릭스 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 주요 개발
- 합병과 인수
- 파트너십 및 협업
- 신제품 발매
제5장 시장 추정·예측 : 제품별(2021-2034년)
- 주요 동향
- 부페닐
- 라빅티
- 암모눌
- 영양보조식품
- 기타 제품
제6장 시장 추정·예측 : 투여 경로별(2021-2034년)
- 주요 동향
- 경구
- 정맥
제7장 시장 추정·예측 : 연령층별(2021-2034년)
- 주요 동향
- 소아
- 성인
제8장 시장 추정·예측 : 유통 채널별(2021-2034년)
- 주요 동향
- 병원 약국
- 소매 약국
- 온라인 약국
제9장 시장 추정·예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 인도
- 일본
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 프로파일
- Abbott Laboratories
- Acer Therapeutics
- Amgen
- Arcturus Therapeutics
- Bausch Health Companies
- Nutricia(Danone Group)
- Mead Johnson(Reckitt Benckiser)
- Nestle
- OrphanPacific
- Ultragenyx Pharmaceutical
The Global Ornithine Transcarbamylase Deficiency Treatment Market was valued at USD 774.2 million in 2024 and is estimated to grow at a CAGR of 4.3% to reach USD 1.2 billion by 2034. Market expansion is being driven by the rising incidence of urea cycle disorders (UCDs) and continued innovation in precision medicine and gene therapies. Increased awareness among healthcare professionals and patients, along with more advanced diagnostic tools, are contributing to higher detection rates and broader treatment needs. A growing number of late-onset cases, particularly in females, is expanding the patient base and fueling demand.
The development of next-generation therapies, including gene editing technologies, enzyme replacement treatments, and liver-targeted small molecules, is being supported by significant investments in research and clinical trials. A favorable regulatory framework, including orphan drug designations, financial incentives, and accelerated approval processes, is further propelling the market forward. This segment of the healthcare industry focuses on therapies that address a rare genetic disorder where the urea cycle is impaired, leading to elevated ammonia levels in the bloodstream, an often life-threatening condition requiring ongoing management.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $774.2 Million |
| Forecast Value | $1.2 Billion |
| CAGR | 4.3% |
The Ravicti segment generated USD 295.7 million in 2024. This segment benefits from strong patient preference due to its ease of use and improved tolerability, especially for children and the elderly. Its user-friendly formulation enhances treatment adherence, a crucial factor in managing chronic, lifelong conditions. The therapy has gained regulatory approval across several regions, increasing its accessibility and uptake across various healthcare systems. Its approval for pediatric use has notably expanded the eligible population, contributing to the segment's overall dominance.
The oral treatments segment held a 62.4% share in 2024. The preference for oral administration stems from its convenience and ability to reduce dependency on frequent hospital visits, which is essential for managing a condition that requires continuous care. Advancements in pharmaceutical formulations, such as extended-release technologies, have improved the therapeutic effectiveness of oral medications, making them comparable to intravenous options. This progress not only enhances patient compliance but also allows for more personalized dosing strategies, contributing to the broader adoption of oral OTC treatments.
United States Ornithine Transcarbamylase Deficiency Treatment Market was valued at USD 315.6 million in 2024. High healthcare spending in the country ensures greater access to treatment options for patients dealing with rare conditions like OTC deficiency. Collaborative efforts among pharmaceutical developers, research institutes, and biotechnology firms are accelerating innovation in this space. Additionally, supportive initiatives such as the Orphan Drug Act are encouraging the development and faster approval of therapies targeting rare diseases. Government funding and incentive programs continue to play a critical role in advancing research and the availability of life-saving treatments.
Key players shaping the Global Ornithine Transcarbamylase Deficiency Treatment Market include Ultragenyx Pharmaceutical, Abbott Laboratories, Bausch Health Companies, Arcturus Therapeutics, Nutricia (Danone Group), Mead Johnson (Reckitt Benckiser), Acer Therapeutics, OrphanPacific, Nestle, and Amgen. To strengthen their market positions, companies in the ornithine transcarbamylase deficiency treatment market are focusing on several strategic initiatives. These include accelerating clinical development of innovative gene therapies and enzyme replacement solutions, investing in partnerships and licensing deals to expand global access, and securing regulatory advantages through orphan drug designations. Many firms are also enhancing patient support programs to increase treatment adherence and building manufacturing capabilities to ensure steady supply chains. Additionally, they are broadening their geographic footprint by targeting emerging markets and aligning with regional health agencies to improve diagnosis and early intervention strategies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.1.1 Key market trends
- 2.1.2 Regional
- 2.1.3 Product
- 2.1.4 Route of administration
- 2.1.5 Age group
- 2.1.6 Distribution channel
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increased awareness for earlier detection of OTC deficiency
- 3.2.1.2 High unmet medical need for life-threatening condition
- 3.2.1.3 Strong insurance and reimbursement by public and private insurers
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Limited availability of approved therapies
- 3.2.2.2 High cost of treatment
- 3.2.3 Industry pitfalls and challenges
- 3.2.3.1 Increasing diagnosis rates due to expanded newborn screening
- 3.2.3.2 Growing availability of specialty pharmacies and rare disease infrastructure
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Future market trends
- 3.6 Pipeline analysis
- 3.7 Porter's analysis
- 3.8 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key development
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New Product Launches
Chapter 5 Market Estimates and Forecast, By Product, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Buphenyl
- 5.3 Ravicti
- 5.4 Ammonul
- 5.5 Dietary supplements
- 5.6 Other products
Chapter 6 Market Estimates and Forecast, By Route of Administration, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Oral
- 6.3 Intravenous
Chapter 7 Market Estimates and Forecast, By Age Group, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Pediatrics
- 7.3 Adults
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospital pharmacies
- 8.3 Retail pharmacies
- 8.4 Online pharmacies
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 India
- 9.4.3 Japan
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott Laboratories
- 10.2 Acer Therapeutics
- 10.3 Amgen
- 10.4 Arcturus Therapeutics
- 10.5 Bausch Health Companies
- 10.6 Nutricia (Danone Group)
- 10.7 Mead Johnson (Reckitt Benckiser)
- 10.8 Nestle
- 10.9 OrphanPacific
- 10.10 Ultragenyx Pharmaceutical













