
|
시장보고서
상품코드
1766297
이종이식 시장 : 기회, 성장 촉진요인, 산업 동향 분석 및 예측(2025-2034년)Xenotransplantation Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
세계의 이종이식 시장 규모는 2024년에 479억 달러로 평가되었고, 2034년에는 1,097억 달러에 달할 것으로 예측되며, CAGR 8.8%로 성장할 전망입니다. 장기 수요와 공급의 불균형이 심화되면서 장기 부족 위기의 장기적인 해결책으로 이종이식에 대한 관심이 다시 고조되고 있습니다. 유전자 편집 기술, 특히 CRISPR/Cas9와 같은 도구들의 발전으로 과학자들은 면역 거부 위험이 낮은 유전자 편집 동물 장기를 연구하고 있습니다. 바이오테크 기업, 벤처 캐피털 그룹, 정부 기관의 강력한 재정적 지원은 엑스노트랜스플랜테이션 혁신의 경계를 확장하고 임상 적용으로의 전환을 가속화하고 있습니다.
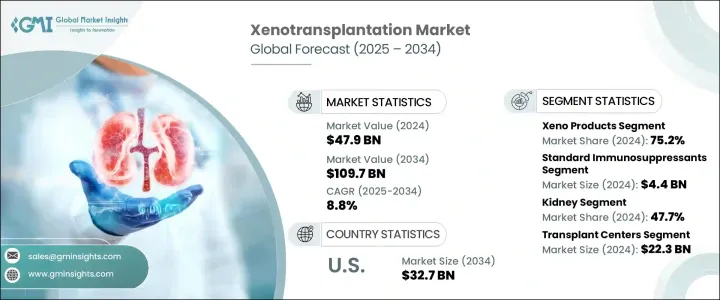
유전자 공학, 고급 생물공학, 인공지능 등 분야의 급속한 기술 혁신은 엑스노트랜스플랜테이션의 미래 잠재력을 재편하고 있습니다. 예측형 인공지능 도구는 장기 매칭과 면역 호환성 추적에 더 큰 역할을 맡으며 이식 성공률을 높이고 거부 반응을 최소화하고 있습니다. 이러한 진전은 엑스노트랜스플랜테이션이 실험 단계에서 일상적인 의료 실천으로 전환되는 전환점을 표시합니다. 전망에는 스마트 시스템, 생체 공학 조직 및 개선된 면역 억제 프로토콜의 통합이 확대되어 종간 장기 이식의 신뢰성과 접근성이 크게 향상될 것으로 보입니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 479억 달러 |
| 예측 금액 | 1,097억 달러 |
| CAGR | 8.8% |
2024년에 Xeno 제품 부문은 75.2%의 점유율을 차지했으며, 이식 대기 시간을 단축하는 역할로 인해 계속해서 우위를 차지할 것으로 보입니다. 유전자 변형 동물에서 추출한 이 제품은 장기 이식을 기다리는 환자에게 실용적인 해결책을 제공합니다. 공학 조직 및 면역 조절 전략의 개선으로 면역 거부 및 공격적인 면역 억제 요법과 관련된 합병증이 감소했습니다. 지원용 생체 재료 및 호환 가능한 조직의 발전은 임상 성공률을 높이고 전 세계 의료 시스템에 대한 채택을 확대할 것으로 예상됩니다.
신장 이식 부문은 2024년에 47.7%의 점유율로 시장을 주도했습니다. 이종이식 노력은 말기 신장 질환(ESRD)의 높은 유병률과 인간 기증자 신장의 부족으로 인해 신장 대체 솔루션에 특히 집중되어 있습니다. 전 세계적으로 약 8억 5천만 명의 사람들이 신장 질환을 앓고 있으며, 특히 의료 서비스가 부족한 저소득 국가에서 그 수가 많기 때문에 이종이식과 같은 대체 솔루션의 필요성이 더욱 시급해지고 있습니다. 유전자 조작 돼지 신장은 이처럼 증가하는 환자군에게 확장 가능하고 생명을 구할 수 있는 치료법입니다.
미국의 이종이식 시장은 2024년에는 138억 달러로 평가되었고, 2034년에는 327억 달러에 이를 것으로 추정되고 있습니다. 기증 장기 공급과 환자 수요 간의 지속적인 격차는 이 국가에서 엑스노트랜스플랜테이션을 의료 혁신의 최전선에 위치시켰습니다. 국가 차원의 자금 지원과 연구 보조금은 장기 보존, 유전자 변형, 이식 임상 시험 등 분야에서 주요 기관들이 개발을 가속화하는 데 기여했습니다. 미국은 이 분야의 전 세계적 리더로 부상하고 있으며, 여러 기관이 이 혁신적인 시장을 촉진하는 임상 발전과 과학적 혁신에 기여하고 있습니다.
세계 이종이식 산업의 미래를 형성하는 주요 기업으로는 XVIVO, Pfizer, Makana Therapeutics, eGenesis, OrganOX, Sanofi, Astellas Pharma, Novartis, Bristol-Myers Squibb Company, United Therapeutics, F. Hoffmann-La Roche, Nzeno 및 Preservation Solutions 등이 있습니다. 이종이식 시장의 선도 기업들은 호환성을 높이고 면역 거부 위험을 줄이기 위해 유전자 변형 기술의 발전에 주력하고 있습니다. 많은 기업들이 혁신을 가속화하고 결과를 검증하기 위해 연구 기관 및 임상 시험 네트워크와의 파트너십을 확대하고 있습니다. 여러 기업들이 면역 프로파일링 및 장기 매칭을 위한 AI 기반 플랫폼에 투자하여 이식 성공률을 높이고 있습니다. 장기 생존율과 환자 치료 결과를 개선하기 위해 전임상 연구 및 생명 공학 이니셔티브에 자금이 전략적으로 투입되고 있습니다.
목차
제1장 조사 방법과 범위
제2장 주요 요약
제3장 산업 고찰
- 생태계 분석
- 산업에 미치는 영향요인
- 성장 촉진요인
- 조직 및 장기 이식 수요 증가
- 유전자 공학의 진보
- 수술 절차의 증가
- 연구 개발 활동에 대한 높은 투자
- 산업의 잠재적 리스크 및 과제
- 엄격한 규제 시나리오
- 숙련된 전문가의 부족
- 시장 기회
- 임상 시험 및 조기 접근 프로그램의 확대
- 특수 면역 억제제 및 거부 반응 억제 치료제의 개발
- 성장 촉진요인
- 성장 가능성 분석
- 규제 상황
- 미국
- 유럽
- 기술
- 세계의 이종이식량 분석(2025-2034년)
- 파이프라인 분석
- 장래 시장 동향
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 소개
- 경쟁 시장 점유율 분석
- 기업 매트릭스 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 이종이식에 있어서의 면역 억제제의 현상과 전망
제5장 시장 추정 및 예측 : 제품 유형별(2021-2034년)
- 주요 동향
- 장기 보존액
- 이식 진단
- Xeno 제품
- 기관
- 세포
- 조직
제6장 시장 추정 및 예측 : 면역 억제제 유형별(2021-2034년)
- 주요 동향
- 표준 면역 억제제
- 칼시뉴린 억제제
- 항증식제
- mTOR 억제제
- 이종이식 특이적 면역 억제제
- 공자극 차단 요법
- 보체 억제제
- B세포와 T세포 모듈레이터
제7장 시장 추정 및 예측 : 용도별(2021-2034년)
- 주요 동향
- 신장
- 심장
- 간
- 폐
- 기타
제8장 시장 추정 및 예측 : 최종 용도별(2021-2034년)
- 주요 동향
- 이식 센터
- 병원
- 기타
제9장 시장 추정 및 예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 이탈리아
- 스페인
- 네덜란드
- 아시아태평양
- 일본
- 중국
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
제10장 기업 프로파일
- Astellas Pharma
- Bristol-Myers Squibb Company
- eGenesis
- F. Hoffmann-La Roche
- Makana Therapeutics
- Novartis
- Nzeno
- OrganOX
- Pfizer
- Preservation Solutions
- Sanofi
- United Therapeutics
- XVIVO
The Global Xenotransplantation Market was valued at USD 47.9 billion in 2024 and is estimated to grow at a CAGR of 8.8% to reach USD 109.7 billion by 2034. The growing mismatch between organ demand and availability has sparked renewed interest in xenotransplantation as a long-term solution to the organ shortage crisis. As technological progress accelerates in genetic modification, particularly through tools like CRISPR/Cas9, scientists are now working with genetically engineered animal organs that demonstrate reduced immune rejection risks. Strong financial backing from biotech firms, venture capital groups, and government agencies is further pushing the boundaries of xenotransplantation innovation and bringing it closer to mainstream clinical adoption.
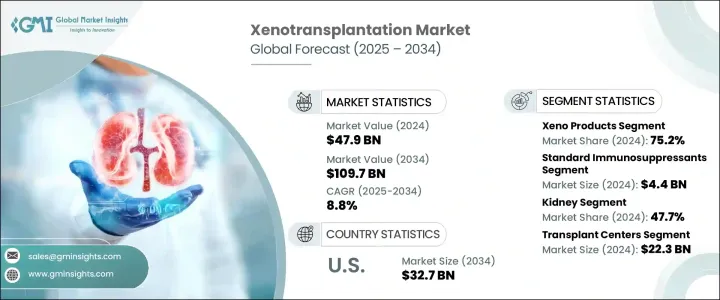
Rapid breakthroughs in fields such as genetic engineering, advanced biotechnology, and artificial intelligence are reshaping xenotransplantation's future potential. Predictive AI tools are playing a larger role in organ matching and immune compatibility tracking, enhancing transplant success rates and minimizing rejection events. Such advancements mark a turning point where xenotransplantation may soon transition from experimental to routine medical practice. The outlook includes the growing integration of smart systems, bioengineered tissues, and improved immunosuppressive protocols, which could significantly increase the reliability and accessibility of cross-species organ transplantation.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $47.9 Billion |
| Forecast Value | $109.7 Billion |
| CAGR | 8.8% |
In 2024, the Xeno products segment generated a 75.2% share and continues to dominate due to its role in easing transplant wait times. These products, derived from genetically altered animal sources, offer a practical solution for patients awaiting organ transplants. Improvements in engineered tissues and immunomodulatory strategies have helped reduce complications related to immune rejection and aggressive immunosuppressive therapy. The evolution of supportive biomaterials and compatible tissues is expected to enhance the clinical success rate and expand adoption across global healthcare systems.
The kidney transplant segment led the market in 2024 with a 47.7% share. Xenotransplantation efforts are particularly focused on kidney replacement solutions due to the high prevalence of end-stage renal disease (ESRD) and the scarcity of human donor kidneys. With an estimated 850 million individuals living with kidney disease globally-especially in lower-income countries with inadequate access to care-the urgency for alternative solutions like xenotransplantation is intensifying. Genetically engineered pig kidneys offer a scalable and potentially life-saving intervention for this expanding patient population.
United States Xenotransplantation Market was valued at USD 13.8 billion in 2024 and is estimated to reach USD 32.7 billion by 2034. The continued gap between donor organ supply and patient needs has placed xenotransplantation at the forefront of medical innovation in the country. National funding and grant support have enabled key institutions to push development forward in organ preservation, genetic modifications, and transplant trials. The country is emerging as a global leader in this field, with several organizations contributing to clinical advancements and scientific breakthroughs that are driving this transformative market.
Key players shaping the landscape of the Global Xenotransplantation Industry include XVIVO, Pfizer, Makana Therapeutics, eGenesis, OrganOX, Sanofi, Astellas Pharma, Novartis, Bristol-Myers Squibb Company, United Therapeutics, F. Hoffmann-La Roche, Nzeno, and Preservation Solutions. Leading companies in the xenotransplantation market are focusing on advancing genetic modification techniques to enhance compatibility and reduce immune rejection risks. Many are expanding partnerships with research institutes and clinical trial networks to accelerate innovation and validate outcomes. Several players are investing in AI-driven platforms for immune profiling and organ matching, increasing transplant success rates. Funding is being strategically directed toward preclinical studies and bioengineering initiatives to improve organ viability and patient outcomes.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.1.1 By product type trends
- 2.1.2 By immunosuppression drug type
- 2.1.3 By application
- 2.1.4 By end use
- 2.1.5 By region
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing demand for tissue and organ transplantation
- 3.2.1.2 Advancements in genetic engineering
- 3.2.1.3 Growing number of surgical procedures
- 3.2.1.4 High investment in research and development activities
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory scenario
- 3.2.2.2 Dearth of skilled professionals
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion of Clinical Trials and Early Access Programs
- 3.2.3.2 Development of Specialized Immunosuppressive and Anti-Rejection Therapies
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.3.1 By product type
- 3.3.2 By immunosuppression drug type
- 3.3.3 By application
- 3.3.4 By end use
- 3.4 Regulatory landscape
- 3.4.1 U.S.
- 3.4.2 Europe
- 3.5 Technology landscape
- 3.6 Global xenotransplants volume analysis, 2025 – 2034
- 3.7 Pipeline analysis
- 3.8 Future market trends
- 3.9 Porter's analysis
- 3.10 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Competitive market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Current and future perspectives on immunosuppressants in xenotransplantation
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 – 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Organ preservation solution
- 5.3 Transplant diagnostics
- 5.4 Xeno products
- 5.4.1 Organ
- 5.4.2 Cell
- 5.4.3 Tissue
Chapter 6 Market Estimates and Forecast, By Immunosuppression Drug Type, 2021 – 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Standard immunosuppressants
- 6.2.1 Calcineurin inhibitors
- 6.2.2 Anti-proliferative agents
- 6.2.3 mTOR inhibitors
- 6.3 Xenotransplant-specific immunosuppressants
- 6.3.1 Costimulation blockade therapy
- 6.3.2 Complement inhibitors
- 6.3.3 B-cell and T-cell modulators
Chapter 7 Market Estimates and Forecast, By Application, 2021 – 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Kidney
- 7.3 Heart
- 7.4 Liver
- 7.5 Lung
- 7.6 Other applications
Chapter 8 Market Estimates and Forecast, By End Use, 2021 – 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Transplant centers
- 8.3 Hospitals
- 8.4 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 – 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Italy
- 9.3.5 Spain
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 Japan
- 9.4.2 China
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.4 Argentina
- 9.6 Middle East and Africa
Chapter 10 Company Profiles
- 10.1 Astellas Pharma
- 10.2 Bristol-Myers Squibb Company
- 10.3 eGenesis
- 10.4 F. Hoffmann-La Roche
- 10.5 Makana Therapeutics
- 10.6 Novartis
- 10.7 Nzeno
- 10.8 OrganOX
- 10.9 Pfizer
- 10.10 Preservation Solutions
- 10.11 Sanofi
- 10.12 United Therapeutics
- 10.13 XVIVO



















