
|
시장보고서
상품코드
1782150
B형 간염 백신 시장 : 시장 기회, 성장 촉진요인, 산업 동향 분석, 예측(2025-2034년)Hepatitis B Vaccine Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034 |
||||||
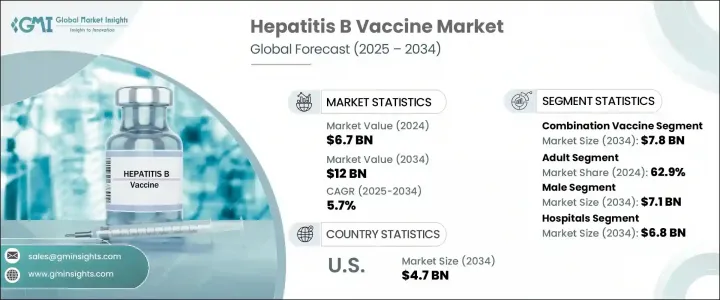
세계의 B형 간염 백신 시장 규모는 2024년에는 67억 달러로 평가되었고, CAGR 5.7%로 성장할 전망이며, 2034년에는 120억 달러에 이를 것으로 추정됩니다.
이 성장 궤도는 세계 B형 간염 환자 수 증가에 크게 영향을 받고 있으며, 정부의 지원에 의한 백신 접종 캠페인과 백신 개발 노력의 가속이 배경에 있습니다. 전국적인 예방 접종 프로그램과 공중보건 의식의 확대로 다양한 연령층에서 백신에 대한 접근과 섭취가 확대되고 있습니다. 게다가, 보다 효율적이고 과녁을 좁힌 솔루션에 대한 수요가, 백신의 제제화와 제조에서 끊임없는 기술 혁신을 뒷받침하고 있습니다. 재조합 기술과 전달 메커니즘의 진보는 더 높은 유효성, 더 오래 지속되는 면역, 필요량의 삭감에 기여하고 있습니다. 헬스케어 시스템이 간 질환 부담 경감을 목표로 하는 가운데, 시장은, 특히 리스크가 높은 사람들 사이에서 예방 백신 접종의 기운이 높아지고 있습니다.
견고하고 신속한 면역 반응을 일으키는 차세대 제형에 대한 선호도가 높아지고 있는 것도 세계의 보급을 뒷받침하고 있습니다. 이들 차세대 백신은 항원의 안정성을 높이고 아주반트 시스템을 개선하여 B형 간염에 대한 예방효과를 보다 신속하고 지속적으로 발휘시키기 위해 전달 방법을 최적화한 것입니다. 이 기술 혁신은 기존 백신으로는 효과적인 반응을 얻을 수 없는 면역 결핍 환자나 고위험 그룹에 특히 중요합니다.
| 시장 범위 | |
|---|---|
| 시작 연도 | 2024년 |
| 예측 연도 | 2025-2034년 |
| 시작 금액 | 67억 달러 |
| 예측 금액 | 120억 달러 |
| CAGR | 5.7% |
2024년에는 혼합 백신 분야가 45억 달러로 시장을 선도하고, 2034년에는 CAGR 5.5%로 성장하면서 78억 달러에 이를 것으로 예측됩니다. 이러한 다가 백신은 B형 간염의 예방과 다른 감염 인자의 예방을 결합하여 필요한 주사 횟수를 줄일 수 있습니다. 이 통합된 접근법은 환자의 컴플라이언스를 향상시키고, 특히 유아와 소아의 예방접종 일정을 간소화합니다. 결과적으로 혼합 백신은 소아 건강 관리에서 널리 채택되어 공공 및 민간 예방 접종 프로그램에 의해 지원됩니다. 행정과 의료 제공업체는 투여 효율성, 대상 범위의 넓이, 비용의 우위로부터 이러한 백신을 선택하는 것이 늘어나고 있어, 지역 전체의 백신 접종율 향상에 공헌하고 있습니다.
남성 분야는 2034년까지 71억 달러에 달할 것으로 예측됩니다. 이것은 남성의 만성 감염과 간 관련 합병증의 위험을 높이는 생물학적 및 호르몬학적 차이로 인한 것이 큽니다. 남성 호르몬은 면역 반응과 바이러스 지속성에 영향을 미치는 것으로 나타났으며, 남성은 B형 간염 감염이 장기화될 위험이 높습니다. 그 결과, 예방접종 프로그램은 성인 남성을 대상으로 되어 왔고, 성인용 백신 제제의 보급도 이 동향을 뒷받침하고 있습니다. 의식 증가, 헬스케어 접근성 개선, 예방 의료 추진으로 이 분야의 백신 보급은 계속 가속화되고 있습니다.
북미의 B형 간염 백신 2024년 시장 규모는 27억 달러로 평가되었고, 2034년까지 CAGR 5.4%로 성장할 전망이며, 2034년에는 47억 달러에 이를 것으로 예측됩니다. 이 지역은 선진적인 헬스케어 제공 시스템과 국민들의 높은 인지도로 세계 B형 간염 백신 시장에서 압도적인 강세를 유지하고 있습니다. 강력한 인프라 및 정부 지원에 의한 광범위한 예방접종 프로그램으로 소아와 성인의 예방접종률은 지속적으로 높은 수준을 유지하고 있습니다. B형 간염은 특히 사회적 약자에게 부담이 여전히 크기 때문에 미국과 캐나다 등 국가에서는 예방 보급과 조기 예방접종 보급을 위해 지속적인 노력이 이루어지고 있습니다.
주요 시장 진출기업은 Serum Institute of India, VBI Vaccines, Sanofi, Biological E, YS Biopharma, GSK, Shenzhen Kangtai Biological Products, Bharat Biotech, Dynavax Technologies, Merck, Gilead Sciences 등입니다. 시장 세분화 분야의 기업은 시장에서의 지위를 강화하기 위해 제품의 혁신, 세계적인 판매망의 확대, 전략적 파트너십에 주력하고 있습니다.
대기업은 안전성 프로파일을 개선하고 면역원성을 강화한 차세대 백신을 개발하기 위해 연구개발에 많은 투자를 하고 있습니다. 많은 기업들은 혼합 백신 플랫폼을 활용함으로써 적용 범위를 확대하고 보다 광범위한 헬스케어 시스템에 어필하고 있습니다. 또, 정부 보건 기관, NGO, 국제 기관과의 제휴에 의해, 신흥 시장에서의 원활한 백신 전개가 가능해지고 있습니다.
목차
제1장 조사 방법 및 범위
제2장 주요 요약
제3장 업계 인사이트
- 생태계 분석
- 공급자의 상황
- 각 단계에서의 부가가치
- 밸류체인에 영향을 주는 요인
- 업계에 미치는 영향요인
- 성장 촉진요인
- 간질환 유병률 상승
- 예방접종 보급 프로그램 확대
- 백신의 처방 및 생산 효율의 진보
- 연구개발 투자 및 활동 증가
- 업계의 잠재적 위험 및 과제
- 엄격한 규제 승인 프로세스
- 백신의 보관 및 운송에 걸리는 고비용
- 시장 기회
- 백신 배포에서 관민 제휴의 확대
- 성인용 백신접종 프로그램의 확대
- 성장 촉진요인
- 성장 가능성 분석
- 파이프라인 분석
- 규제 상황
- 기술적 진보
- 장래 시장 동향
- Porter's Five Forces 분석
- PESTEL 분석
제4장 경쟁 구도
- 서문
- 기업 매트릭스 분석
- 기업의 시장 점유율 분석
- 주요 시장 기업의 경쟁 분석
- 경쟁 포지셔닝 매트릭스
- 주요 발전
- 합병 및 인수
- 파트너십 및 협업
- 확장 계획
제5장 시장 추계 및 예측 : 백신 유형별(2021-2034년)
- 주요 동향
- 단일 항원 백신
- 혼합 백신
제6장 시장 추계 및 예측 : 연령별(2021-2034년)
- 주요 동향
- 소아
- 성인
제7장 시장 추계 및 예측 : 남녀별(2021-2034년)
- 주요 동향
- 여성
- 남성
제8장 시장 추계 및 예측 : 최종 용도별(2021-2034년)
- 주요 동향
- 병원
- 퍼블릭
- 프라이빗
- 전문 클리닉
- 기타 용도
제9장 시장 추계 및 예측 : 지역별(2021-2034년)
- 주요 동향
- 북미
- 미국
- 캐나다
- 유럽
- 독일
- 영국
- 프랑스
- 스페인
- 이탈리아
- 네덜란드
- 아시아태평양
- 중국
- 일본
- 인도
- 호주
- 한국
- 라틴아메리카
- 브라질
- 멕시코
- 아르헨티나
- 중동 및 아프리카
- 남아프리카
- 사우디아라비아
- 아랍에미리트(UAE)
제10장 기업 프로파일
- Bharat Biotech
- Biological E
- Dynavax Technologies
- Gilead Sciences
- GSK
- Merck
- Sanofi
- Serum Institute of India
- Shenzhen Kangtai Biological Products
- VBI vaccines
- YS Biopharma
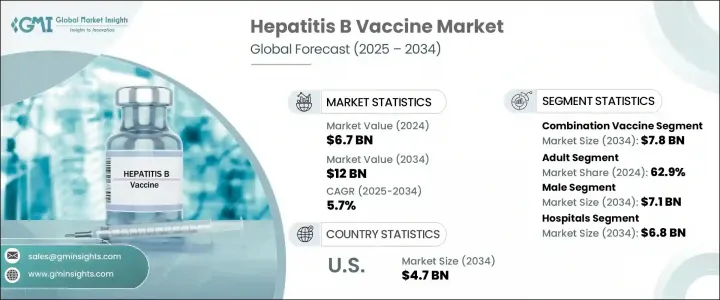
The Global Hepatitis B Vaccine Market was valued at USD 6.7 billion in 2024 and is estimated to grow at a CAGR of 5.7% to reach USD 12 billion by 2034. The growth trajectory is largely influenced by the increasing number of hepatitis B cases worldwide, backed by government-backed vaccination campaigns and accelerated vaccine development efforts. National immunization programs and expanded public health awareness have led to greater access and uptake of vaccines across various age groups. Additionally, demand for more efficient and targeted solutions is pushing continuous innovation in vaccine formulation and manufacturing. Advances in recombinant technology and delivery mechanisms are contributing to higher efficacy, longer-lasting immunity, and reduced dosage requirements. As healthcare systems aim to reduce the burden of liver-related diseases, the market is witnessing stronger momentum for preventive vaccination, particularly among at-risk populations.
The rising preference for next-generation formulations that deliver robust and rapid immune responses further supports adoption globally. These advanced vaccines are being engineered with enhanced antigen stability, improved adjuvant systems, and optimized delivery methods to generate quicker and more sustained protection against hepatitis B. Unlike traditional formulations, next-gen vaccines aim to reduce the number of doses required for full immunity, making them more suitable for large-scale immunization campaigns, especially in regions with limited healthcare access. This innovation is particularly crucial for immunocompromised individuals and high-risk groups who may not respond effectively to conventional vaccines.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $6.7 Billion |
| Forecast Value | $12 Billion |
| CAGR | 5.7% |
In 2024, the combination vaccines segment led the market with a value of USD 4.5 billion and is forecasted to reach USD 7.8 billion by 2034, growing at a CAGR of 5.5%. These multivalent vaccines combine hepatitis B protection with immunization against other infectious agents, reducing the number of injections required. This integrated approach improves patient compliance and simplifies vaccine schedules, especially for infants and children. As a result, combination vaccines have gained widespread adoption in pediatric healthcare, supported by both public and private immunization programs. Governments and providers increasingly opt for these vaccines due to their efficiency in administration, broader coverage, and cost advantages, helping raise vaccination rates across communities.
The male segment is expected to reach USD 7.1 billion by 2034. This is largely driven by biological and hormonal differences that increase the risk of chronic infection and liver-related complications in men. Testosterone has been shown to influence immune response and virus persistence, placing males at a greater risk of prolonged hepatitis B infection. As a result, immunization programs have increasingly focused on adult men, with widespread availability of adult vaccine formulations supporting this trend. Growing awareness, better access to healthcare, and a push for preventive care continue to accelerate vaccine adoption in this segment.
North America Hepatitis B Vaccine Market was valued at USD 2.7 billion in 2024 and is projected to reach USD 4.7 billion by 2034 at a CAGR of 5.4% through 2034. The region remains a dominant force in the global hepatitis B vaccine landscape due to its advanced healthcare delivery systems and high public awareness. Strong infrastructure, along with extensive government-backed vaccination programs, has enabled both pediatric and adult immunization rates to remain consistently high. The burden of hepatitis B remains significant, especially in vulnerable populations, prompting sustained efforts toward widespread prevention and early immunization coverage in countries like the U.S. and Canada.
Key market players include Serum Institute of India, VBI Vaccines, Sanofi, Biological E, YS Biopharma, GSK, Shenzhen Kangtai Biological Products, Bharat Biotech, Dynavax Technologies, Merck, and Gilead Sciences. To strengthen their market position, companies in the hepatitis B vaccine segment are focusing on product innovation, expanding their global distribution networks, and engaging in strategic partnerships.
Leading players are investing heavily in R&D to develop next-generation vaccines with improved safety profiles and stronger immunogenicity. Many are leveraging combination vaccine platforms to increase coverage and appeal to broader healthcare systems. Collaborations with government health agencies, NGOs, and international organizations are also enabling smoother vaccine rollouts in emerging markets.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional
- 2.2.2 Vaccine type
- 2.2.3 Age group
- 2.2.4 Gender
- 2.2.5 End use
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factors affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising prevalence of liver diseases
- 3.2.1.2 Growing immunization coverage programs
- 3.2.1.3 Advancements in vaccine formulation and production efficiency
- 3.2.1.4 Increasing R&D investment and activities
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Stringent regulatory approval processes
- 3.2.2.2 High cost of storage and transportation of vaccine
- 3.2.3 Market opportunities
- 3.2.3.1 Growing public-private partnerships for vaccine distribution
- 3.2.3.2 Expanding adult vaccination programs
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Pipeline analysis
- 3.5 Regulatory landscape
- 3.6 Technological advancements
- 3.7 Future market trends
- 3.8 Porter’s analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 Expansion plans
Chapter 5 Market Estimates and Forecast, By Vaccine Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Single antigen vaccine
- 5.3 Combination vaccine
Chapter 6 Market Estimates and Forecast, By Age Group, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Pediatric
- 6.3 Adult
Chapter 7 Market Estimates and Forecast, By Gender, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Female
- 7.3 Male
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.2.1 Public
- 8.2.2 Private
- 8.3 Specialty clinics
- 8.4 Other end use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Bharat Biotech
- 10.2 Biological E
- 10.3 Dynavax Technologies
- 10.4 Gilead Sciences
- 10.5 GSK
- 10.6 Merck
- 10.7 Sanofi
- 10.8 Serum Institute of India
- 10.9 Shenzhen Kangtai Biological Products
- 10.10 VBI vaccines
- 10.11 YS Biopharma



















