|
시장보고서
상품코드
1597043
무바늘 약물전달 기기 시장 : 산업 동향 및 예측(-2035년) - 기기 유형별, 작용기전별, 제품 사용성별, 부하 유형별, 투여 경로별, 적용 분야별, 제품 유형별, 사용 재료 유형별, 중재 유형별, 지역별Needle Free Drug Delivery Devices Market: Industry Trends and Forecasted Estimates, till 2035 - Distribution by Type of Device, Route of Administration, Application Area, Product Type, Type of Material Used, Type of Intervention and Geography |
||||||
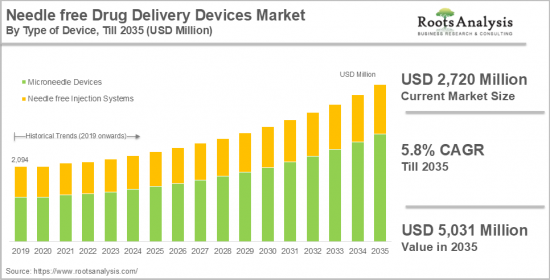
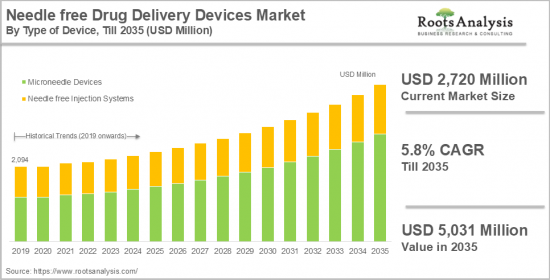
무바늘 약물전달 기기 시장은 무바늘 주사 시스템과 마이크로 니들 기기로 구분됩니다. 니들 프리 약물 주입 시스템 시장은 2024년 6억 600만 달러로 평가되며 2035년까지 예측 기간 동안 4.0%의 CAGR로 확대될 것으로 예상됩니다. 반면, 마이크로니들 디바이스 시장은 2024년 21억 1,400만 달러로 평가되며 2035년까지 예측 기간 동안 6.2%의 CAGR로 성장할 것으로 예상됩니다.
최근 당뇨병, 자가면역질환, 심혈관질환, 종양성 질환 등 다양한 만성질환의 발병률이 크게 증가하고 있습니다. 실제로 University of Michigan의 Center for Managing Chronic Disease에 따르면, 전 세계 인구의 50% 이상이 만성질환을 앓고 있다고 합니다. 특히, 만성질환은 높은 비용과 시간이 지날수록 증가하는 합병증으로 인해 전 세계적으로 사망과 장애의 주요 원인이 되고 있습니다. 이에 따라 만성질환의 증가에 대응하기 위해 혁신적인 전략과 환자 중심의 접근법을 개발해야 할 필요성이 대두되고 있습니다.
현재 만성질환의 치료 방법에는 경구 및 비경구 투여가 있습니다. 약물 투여 경로의 선택은 약물의 특성, 환자의 상태, 확실한 치료 효과의 필요성 등의 요인에 영향을 받습니다. 약물의 비경구 투여는 빠른 작용 발현, 정확한 용량 조절, 높은 생체 이용률로 인해 널리 사용되는 투여 경로입니다. 이러한 장점에도 불구하고 기존의(비경구) 약물전달 방식에는 교차 오염 위험, 주사침 손상, 부정확한 투약 등 여러 가지 문제점이 있습니다. 이러한 문제들은 복약 순응도를 저해하고 궁극적으로 치료 효과에 영향을 미칠 수 있습니다.
시간이 지남에 따라 제약 업계는 앞서 언급한 문제를 해결하기 위한 기술적 진보를 목격했습니다. 이러한 기술 발전의 결과로 안전 주사기, 사용 후 자동으로 바늘을 빼낼 수 있는 안전 주사바늘 등 보다 안전한 대체품이 도입되었습니다. 또한, 각 업체들은 니들 프리 시스템, 제트 인젝터, 펜형 인젝터, 마이크로 니들 패치 등 약물전달 장비의 개발을 진행해왔습니다. 디자인, 기능 및 사용 편의성이 개선된 이들 기기는 바늘에 찔리는 부상의 가능성을 줄여 통증 없이 진피를 통해 약물을 쉽게 자가 투여할 수 있도록 돕습니다. 전통적인 약물전달에서 사용자 친화적인 약물전달 옵션으로의 전환을 고려할 때, 세계 무바늘 약물전달 기기 시장은 예측 기간 동안 큰 성장을 보일 것으로 예상됩니다.
세계 무바늘 약물전달 기기 시장에 대해 조사했으며, 시장 개요와 함께 기기 유형별, 작용 기전별, 제품 사용성별, 부하 유형별, 투여 경로별, 적용 분야별, 제품 유형별, 사용 재료 유형별, 중재 유형별, 지역별 동향, 진출 기업 프로파일을 정리하여 전해드립니다.
목차
제1장 서문
제2장 조사 방법
제3장 시장 역학
제4장 경제 및 기타 프로젝트 특유의 고려사항
제5장 주요 요약
제6장 소개
제7장 시장 상황 : 무바늘 주사 시스템
제8장 시장 상황 : 마이크로니들 디바이스
제9장 제품 경쟁력 분석 : 무바늘 주사 시스템
제10장 제품 경쟁력 분석 : 마이크로니들 디바이스
제11장 유망한 약제 후보 : 무바늘 주사 시스템
제12장 기업 개요 : 북미를 거점으로 하는 무바늘 약물전달 기기 프로바이더
제13장 기업 개요 : 유럽을 거점으로 하는 무바늘 약물전달 기기 프로바이더
제14장 기업 개요 : 아시아태평양 및 기타 지역에 거점을 두는 무바늘 약물전달 기기 프로바이더
제15장 메가트렌드 분석
제16장 시장 영향 분석
- 분석 개요
- 시장 촉진요인
- 시장 억제요인
- 시장 기회
- 시장 과제
- 결론
제17장 세계의 무바늘 주사 시스템 시장
제18장 무바늘 주사 시스템 시장(제품 유형별)
제19장 무바늘 주사 시스템 시장(작용기서별)
제20장 무바늘 주사 시스템 시장(제품 사용성별)
제21장 무바늘 주사 시스템 시장(부하 유형별)
제22장 무바늘 주사 시스템 시장(투여 경로별)
제23장 무바늘 주사 시스템 시장(적용 영역별)
제24장 무바늘 주사 시스템 시장(지역별)
제25장 세계의 마이크로니들 디바이스 시장
제26장 마이크로니들 디바이스 시장(마이크로니들 유형별)
제27장 마이크로니들 디바이스 시장(사용 재료별)
제28장 마이크로니들 디바이스 시장(용도별)
제29장 마이크로니들 디바이스 시장(개입 유형별)
제30장 마이크로니들 디바이스 시장(지역별)
제31장 결론
제32장 이그제큐티브 인사이트
제33장 부록 I : 표형식 데이터
제34장 부록 II : 기업 및 단체 리스트
ksm 24.11.29Needle free drug delivery devices market is segmented into needle free injection systems and microneedle devices. The needle-free drug injection systems market is valued at USD 606 million in 2024 and is projected to grow at a CAGR of 4.0% during the forecast period, till 2035. While the microneedle devices market is valued at USD 2,114 million in 2024, growing at a CAGR of 6.2% during the forecast period, till 2035.
Recent years have witnessed a significant increase in incidence rates of various chronic diseases, such as diabetes, autoimmune diseases, cardiovascular diseases, and oncological diseases. In fact, according to the Center for Managing Chronic Disease at the University of Michigan, over 50% of the global population is suffering from some form of chronic disease. Notably, these conditions are the leading cause of death and disability worldwide, due to high cost and associated complications that tend to escalate over time. This has necessitated the need to develop innovative strategies and patient centered approaches in order to tackle the increasing burden of chronic diseases.
Presently, the treatment options available for chronic conditions include administration of drugs via oral and parenteral routes. The choice of route for medication administration is influenced by factors such as drug properties, patient condition, and the need for reliable therapeutic effect. Since, parenteral administration of drugs involves rapid onset of action, precise dosing control and higher bioavailability, it is the widely accepted route of administration. Despite these advantages offered by the conventional (parenteral) drug delivery approaches, there are various challenges associated with parenteral administration, including risk of cross contamination, needlestick injuries, and inaccurate dosing. These challenges further lead to hindrance in medication adherence and ultimately impact the therapeutic outcomes.
Over time, the pharmaceutical industry has witnessed technological breakthroughs in order to counter the aforementioned challenges. These technological advancements have resulted in the introduction of safer alternatives, such as safety syringes and safety needles that allow automatic retraction of needles after use. In addition, the companies have advanced the development of drug delivery devices which include needle free systems, jet injectors, pen injectors and microneedle patches. With improved design, functionality and usability, these devices reduce the possibility of needlestick injuries, thereby facilitating pain-free and self-administration of medications across the dermis. Given this transition from conventional to user-friendly drug delivery options, the global needle free drug delivery devices market is expected to experience significant growth during the forecast period.
Key Market Segments
Route of Administration (Needle free drug delivery devices)
- Subcutaneous
- Intramuscular
- Intradermal
Actuation Mechanism (Needle free drug delivery devices)
- Spring-powered Devices
- Compressed-gas Devices
- Other Devices
Application Area (Needle free drug delivery devices)
- Insulin Delivery
- Pain Management
- Dermatology
- Vaccination
Product Type (Needle free drug delivery devices)
- Fillable Devices
- Prefilled Devices
Type of Load (Needle free drug delivery devices)
- Liquid-based Devices
- Powder-based Devices
- Projectile/Depot-based Devices
Geography (Needle free drug delivery devices)
- North America (US and Canada)
- Europe (Germany, UK, France, Italy and Spain)
- Asia-Pacific (India, China, Japan and Australia)
- Middle East and North Africa (Saudi Arabia, Egypt and Israel)
- Latin America Africa (Brazil, Argentina and Mexico)
Type of Microneedle (Microneedle devices)
- Hollow Microneedles
- Solid Microneedles
- Dissolving Microneedles
- Coated Microneedles
Application Area (Microneedle devices)
- Insulin Delivery
- Pain Management
- Vaccination
- Oncology
Type of Material Used (Microneedle devices)
- Polymer Microneedle
- Silicon Microneedle
- Metal Microneedle
- Ceramic Microneedle
Type of Intervention (Microneedle devices)
- Vaccines
- Therapeutic Agents
- Other Interventions
Geography (Microneedle devices)
- North America (US and Canada)
- Europe (France, Germany, Italy, Spain, UK and Rest of Europe)
- Asia-Pacific (India, China, Japan, Australia and Rest of Asia-Pacific)
- Middle East and North Africa (Saudi Arabia, Egypt and Israel)
- Latin America Africa (Brazil, Argentina and Mexico)
Research Coverage:
The report on needle free drug delivery devices market provides insights into various aspects, including:
- A preface introducing the full report, Needle Free Drug Delivery Devices Market, till 2035.
- A detailed outline of the structured research methodology used in the study, covering research assumptions, project and forecast methodologies, primary and secondary research approaches, along with the various analytical frameworks applied.
- A summary of the diverse methodologies and frameworks used to forecast and analyze market trends, highlighting the core factors affecting dynamics. It also underlines the stringent quality control framework employed to maintain transparency and reliability throughout the report.
- A brief analysis of the economic variables affecting the needle free drug delivery devices market, such as currency fluctuations, foreign exchange rates, and known trade barriers. Additionally, it considers the impact of global recession and inflation using historical trends to project future market conditions.
- An executive summary that provides the insights derived from our research, presenting key takeaways from the current market landscape of needle free drug delivery devices. The section also provides details on market evolution in the short to mid to long term.
- A brief introduction to needle free injection systems and microneedles, highlighting the growing demand for devices that enable painless administration of medication in the home healthcare setting. The section emphasizes the need for such devices, specifically in terms of the rising incidence and prevalence of chronic diseases. Subsequently, it provides an overview of the different types of needle free injectors and microneedles, detailing their specifications, mechanisms of action, R&D challenges, and expected future trends.
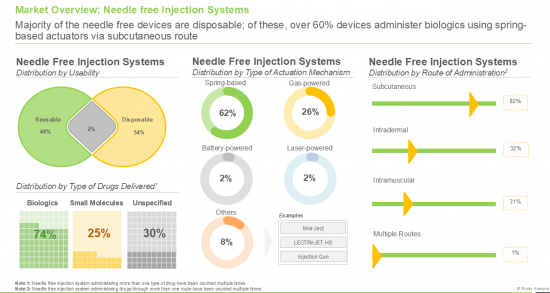
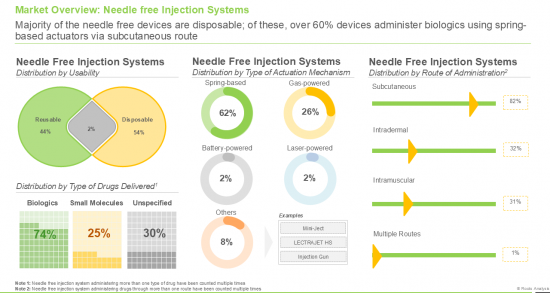
- A comprehensive assessment of the overall market landscape of needle free drug delivery providers, based on several relevant parameters including year of establishment, company size (based on the employee count), location of their headquarters (region and country), actuation mechanism (spring-powered, gas-powered, battery-powered, laser-powered and others), route of administration (subcutaneous, intradermal, intramuscular and multiple), type of formulation administered (liquids, solids and powders), type of drug delivered (biologics, small molecules and unspecified), therapeutic area (metabolic disorders, dermatological disorders, infectious diseases, musculoskeletal disorders, neurological disorders, autoimmune / inflammatory disorders, genetic disorders, ophthalmological disorders and renal disorders), and usability (reusable and disposable).
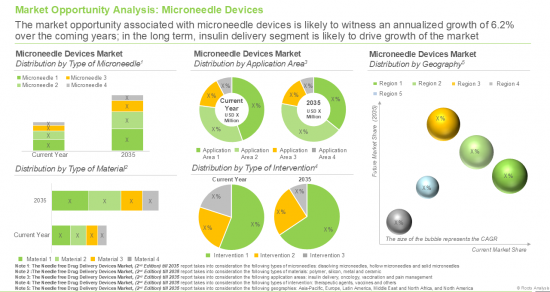
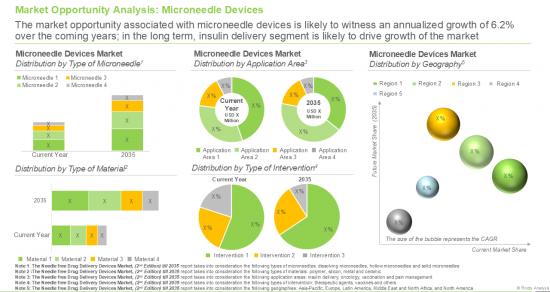
- A detailed assessment of the overall market landscape of microneedle device providers, based on several relevant parameters. The parameters include year of establishment, company size (based on the employee count), location of their headquarters (region and country), stage of development (marketed / under development), type of microneedle (hollow, solid and dissolving), type of formulation administered (liquids, solids and powders), route of administration (intradermal, transdermal, subcutaneous, topical, intraocular, trans buccal, intravenous, subretinal and intravitreal) therapeutic area (metabolic disorders, dermatological disorders, infectious diseases, oncological disorders, musculoskeletal disorders, neurological disorders, cardiovascular disorders, ophthalmic disorders and others) and type of drug delivered (biologics, small molecules and unspecified).
- A detailed product competitiveness analysis of needle-free injection systems, based on the supplier power and product specifications. The analysis allows the companies providing needle-free injection systems to compare their existing capabilities and identify opportunities to achieve a competitive edge in the industry.
- A detailed product competitiveness analysis of microneedle devices, based on the supplier power and product specifications. The analysis enables microneedle device providers to benchmark their existing capabilities and identify opportunities to achieve a competitive edge in the industry.
- A detailed likely drug candidate analysis presenting information on the list of marketed drugs / therapies and pipeline candidates that are anticipated to be developed in combination with needle free injectors in the near future. The analysis is based on a variety of relevant parameters including (in alphabetical order) current status of development, dose concentration, dosing frequency, route of administration, type of dose (standard / weight dependent), expected patent expiry (relevant only for marketed drugs) and information on product sales (relevant only for marketed drugs).
- A detailed likely drug candidate analysis presenting information on the list of marketed drugs/ therapies and pipeline candidates that are anticipated to be developed in combination with microneedles in the near future. The analysis is based on a variety of relevant parameters, such as (in alphabetical order) current status of development, dose concentration, dosing frequency, route of administration, type of dose (standard / weight dependent), expected patent expiry (relevant only for marketed drugs) and information on product sales (relevant only for marketed drugs).
- An elaborate set of profiles of prominent companies in needle free drug delivery devices market headquartered in North America, Europe, Asia-Pacific and rest of the world. Each profile provides a brief overview of the company, along with information on its product portfolio, and an insightful recent development and future outlook.
- A comprehensive outlook of the various ongoing megatrends in the needle free drug delivery devices market, including technological advancements in design, functionality, usability and features, along with expanded applications of needle free systems and increasing focus on personalized medicine.
- A detailed market impact analysis providing details on the factors that can impact the growth of needle free drug delivery devices market. It features insights on key drivers, potential restraints, emerging opportunities, and existing challenges in this industry.
- A comprehensive evaluation of the current market size and future market growth potential of the needle-free injection system market (first product category within the needle free drug delivery devices market) over the next 11 years. On the basis of multiple parameters, likely adoption trends and through primary validations, we have provided an informed estimate on the market size, till 2035.
- Detailed forecast projections of the current and future opportunity within the needle-free injection system market across different product types, including fillable devices and prefilled devices.
- Detailed forecast projections of the current and future opportunity within the needle-free injection system market across different actuation mechanisms, including spring-powered devices, compressed-gas devices and other devices.
- Detailed forecast projections of the current and future opportunity within the needle-free injection system market across product usability, including reusable devices and disposable devices.
- Detailed forecast projections of the current and future opportunity within the needle-free injection system market across type of load, including liquid-based devices, powder-based devices and projectile / depot-based devices.
- Detailed forecast projections of the current and future opportunity within the needle-free injection system market across different routes of administration, including subcutaneous, intramuscular and intradermal.
- Detailed forecast projections of the current and future opportunity within the needle-free injection system market across application area, including insulin delivery, pain management, vaccination and dermatology.
- Detailed forecast projections of the current and future opportunity within the needle-free injection system market across key geographical regions including North America, Europe, Asia-Pacific, Middle East and North Africa, and Latin America.
- A comprehensive assessment of the current microneedle market size (second product category within the needle free drug delivery devices market), along with the future market growth potential over the next 11 years. On the basis of several relevant parameters including likely adoption trends and through primary validations, we have provided an informed estimate on the market size till 2035.
- A detailed evaluation of the current and future opportunity within the microneedle device market (second product category within the needle free drug delivery devices market) segmented by different types of microneedles, such as hollow microneedles, solid microneedles, dissolving microneedles and coated microneedles.
- A detailed evaluation of the current and future opportunity within the microneedle device market segmented by different types of material used, such as polymer microneedles, silicon microneedles, metal microneedles and ceramic microneedles.
- A detailed evaluation of the current and future opportunity within the microneedle device market segmented by different application areas, such as insulin delivery, vaccination, pain management and oncology.
- A detailed evaluation of the current and future opportunity within the microneedle device market segmented by different types of intervention, such as vaccines, therapeutic agents and other interventions.
- A detailed evaluation of the current and future opportunity within the microneedle device market segmented by key geographical regions including North America, Europe, Asia-Pacific, Middle East and North Africa, and Latin America.
Key Benefits of Buying this Report
- The report provides valuable insights to market leaders and newcomers related to the revenue estimations of the overall market and its sub-segments.
- Stakeholders can utilize the report to enhance their understanding of the competitive landscape, allowing for improved business positioning and more effective go-to-market strategies.
- The report provides stakeholders with a comprehensive outlook of the needle free drug delivery devices market, furnishing them with essential information on significant market drivers, barriers, opportunities, and challenges.
Example Companies Profiled
- 3M
- Debiotech
- INCYTO
- Micropoint Technologies
- NanoPass Technologies
- Nemaura Pharma
- Theraject
- Antares Pharma
- D'Antonio Consultants International
- HNS International
- Injex
- INOVIO Pharmaceuticals
- Medical International Technology
- Mika Medical
- Pharmajet
- QS Medical Technology (Quinovare)
- Zogenix
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Market Share Insights
- 1.3. Key Market Insights
- 1.4. Report Coverage
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.2.1. Market Landscape and Market Trends
- 2.2.2. Market Forecast and Opportunity Analysis
- 2.2.3. Comparative Analysis
- 2.3. Database Building
- 2.3.1. Data Collection
- 2.3.2. Data Validation
- 2.3.3. Data Analysis
- 2.4. Project Methodology
- 2.4.1. Secondary Research
- 2.4.1.1. Annual Reports
- 2.4.1.2. Academic Research Papers
- 2.4.1.3. Company Websites
- 2.4.1.4. Investor Presentations
- 2.4.1.5. Regulatory Filings
- 2.4.1.6. White Papers
- 2.4.1.7. Industry Publications
- 2.4.1.8. Conferences and Seminars
- 2.4.1.9. Government Portals
- 2.4.1.10. Media and Press Releases
- 2.4.1.11. Newsletters
- 2.4.1.12. Industry Databases
- 2.4.1.13. Roots Proprietary Databases
- 2.4.1.14. Paid Databases and Sources
- 2.4.1.15. Social Media Portals
- 2.4.1.16. Other Secondary Sources
- 2.4.2. Primary Research
- 2.4.2.1. Types of Primary Research
- 2.4.2.1.1. Qualitative Research
- 2.4.2.1.2. Quantitative Research
- 2.4.2.1.3. Hybrid Approach
- 2.4.2.2. Advantages of Primary Research
- 2.4.2.3. Techniques for Primary Research
- 2.4.2.3.1. Interviews
- 2.4.2.3.2. Surveys
- 2.4.2.3.3. Focus Groups
- 2.4.2.3.4. Observational Research
- 2.4.2.3.5. Social Media Interactions
- 2.4.2.4. Key Opinion Leaders Considered in Primary Research
- 2.4.2.4.1. Company Executives (CXOs)
- 2.4.2.4.2. Board of Directors
- 2.4.2.4.3. Company Presidents and Vice Presidents
- 2.4.2.4.4. Research and Development Heads
- 2.4.2.4.5. Technical Experts
- 2.4.2.4.6. Subject Matter Experts
- 2.4.2.4.7. Scientists
- 2.4.2.4.8. Doctors and Other Healthcare Providers
- 2.4.2.5. Ethics and Integrity
- 2.4.2.5.1. Research Ethics
- 2.4.2.5.2. Data Integrity
- 2.4.2.1. Types of Primary Research
- 2.4.3. Analytical Tools and Databases
- 2.4.1. Secondary Research
3. MARKET DYNAMICS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Forecasted Estimates
- 3.2.2. Currency Coverage
- 3.2.2.1. Overview of Major Currencies Affecting the Market
- 3.2.2.2. Impact of Currency Fluctuations on the Industry
- 3.2.3. Foreign Exchange Impact
- 3.2.3.1. Evaluation of Foreign Exchange Rates and Their Impact on Market
- 3.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 4.1. Chapter Overview
- 4.2. Market Dynamics
- 4.2.1. Time Period
- 4.2.1.1. Historical Trends
- 4.2.1.2. Current and Forecasted Estimates
- 4.2.2. Currency Coverage
- 4.2.2.1. Overview of Major Currencies Affecting the Market
- 4.2.2.2. Impact of Currency Fluctuations on the Industry
- 4.2.3. Foreign Exchange Impact
- 4.2.3.1. Evaluation of Foreign Exchange Rates and Their Impact on Market
- 4.2.3.2. Strategies for Mitigating Foreign Exchange Risk
- 4.2.4. Recession
- 4.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 4.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 4.2.5. Inflation
- 4.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 4.2.5.2. Potential Impact of Inflation on the Market Evolution
- 4.2.1. Time Period
5. EXECUTIVE SUMMARY
6. INTRODUCTION
- 6.1. Chapter Overview
- 6.2. Historical Evolution of Drug Delivery Devices
- 6.3. Conventional Parenteral Delivery
- 6.3.1. Needlestick Injuries
- 6.3.1.1. Incidence Rate and Cost Burden of Needlestick Injuries
- 6.3.1. Needlestick Injuries
- 6.4. Minimally Invasive Drug Delivery
- 6.4.1. Factors Influencing Growth of Minimally Invasive Drug Delivery Systems
- 6.4.1.1. Rising Burden of Chronic Diseases
- 6.4.1.2. Healthcare Cost Savings
- 6.4.1.3. Need for Immediate Treatment in Emergency Situations
- 6.4.1.4. Growing Injectable Drugs Market
- 6.4.1.5. Need for Improving Medication Adherence
- 6.4.1. Factors Influencing Growth of Minimally Invasive Drug Delivery Systems
- 6.5. Needle-free Injection Technology
- 6.5.1. Key Components of Needle-free Injection Systems
- 6.5.1.1. Injection Device
- 6.5.1.2. Nozzle
- 6.5.1.3. Pressure Source
- 6.5.1. Key Components of Needle-free Injection Systems
- 6.6. Operating Mechanism of Needle-free Injection Systems
- 6.7. Classification based on Type of Load
- 6.7.1. Powder-based Injectors
- 6.7.2. Liquid-based Injectors
- 6.7.3. Depot or Projectile Injectors
- 6.8. Classification based on Actuation Mechanism
- 6.8.1. Spring Loaded Jet Injectors
- 6.8.2. Battery Powered Jet Injectors
- 6.8.3. Gas Powered Jet Injectors
- 6.8.4. Laser Powered Injectors
- 6.8.5. Lorentz Force-based Injectors
- 6.9. Microneedle Devices
- 6.9.1. Types of Microneedle Devices
- 6.9.2. Advantages of Microneedle Devices
- 6.9.3. Operating Mechanism of Microneedle Devices
- 6.10. Key Challenges related to Needle-free Injection Systems and Microneedle Devices
- 6.11. Recent Advancements
- 6.12. Future Perspectives
7. MARKET LANDSCAPE: NEEDLE-FREE INJECTION SYSTEMS
- 7.1 Chapter Overview
- 7.2. Needle-free Injection System Providers: Overall Market Landscape
- 7.2.1. Analysis by Year of Establishment
- 7.2.2. Analysis by Company Size
- 7.2.3. Analysis by Location of Headquarters
- 7.2.4. Analysis by Company Size and Location of Headquarters
- 7.3. Needle-free Injection Systems: Overall Market Landscape
- 7.3.1. Analysis by Actuation Mechanism
- 7.3.2. Analysis by Route of Administration
- 7.3.3. Analysis by Type of Formulation Administered
- 7.3.4. Analysis by Type of Drug Delivered
- 7.3.5. Analysis by Therapeutic Area
- 7.3.6. Analysis by Usability
- 7.3.7. Analysis by Type of Formulation Administered and Actuation Mechanism
8. MARKET LANDSCAPE: MICRONEEDLE DEVICES
- 8.1 Chapter Overview
- 8.2. Microneedle Device Providers: Overall Market Landscape
- 8.2.1. Analysis by Year of Establishment
- 8.2.2. Analysis by Company Size
- 8.2.3. Analysis by Location of Headquarters
- 8.2.4. Analysis by Company Size and Location of Headquarters
- 8.3. Microneedle Devices: Overall Market Landscape
- 8.3.1. Analysis by Stage of Development
- 8.3.2. Analysis by Type of Microneedle
- 8.3.3. Analysis by Type of Formulation Administered
- 8.3.4. Analysis by Route of Administration
- 8.3.5. Analysis by Therapeutic Area
- 8.3.6. Analysis by Type of Drug Delivered
- 8.3.7. Analysis by Type of Microneedle and Type of Formulation Administered
9. PRODUCT COMPETITIVENESS ANALYSIS: NEEDLE-FREE INJECTION SYSTEMS
- 9.1 Chapter Overview
- 9.2. Assumptions and Key Parameters
- 9.3. Methodology
- 9.4. Product Competitiveness Analysis: Needle-free Injection Systems
- 9.4.1. Spring-based Needle-free Injection Systems
- 9.4.2. Gas-powered Needle-free Injection Systems
- 9.4.3. Other Needle-free Injection Systems
10. PRODUCT COMPETITIVENESS ANALYSIS: MICRONEEDLE DEVICES
- 10.1 Chapter Overview
- 10.2. Assumptions and Key Parameters
- 10.3. Methodology
- 10.4. Product Competitiveness Analysis: Microneedle Devices
- 10.4.1. Dissolving Microneedle Devices
- 10.4.2. Hollow Microneedle Devices
- 10.4.3. Solid Microneedle Devices
- 10.4.4. Other Microneedle Devices
11. LIKELY DRUG CANDIDATES: NEEDLE-FREE INJECTION SYSTEMS
- 11.1 Chapter Overview
- 11.2. Marketed Drugs Candidates
- 11.2.1. Most Likely Candidates for Delivery via Needle-Free Injection Systems
- 11.2.2. Likely Candidates for Delivery via Needle-Free Injection Systems
- 11.2.3. Less Likely Candidates for Delivery via Needle-Free Injection Systems
- 11.2.4. Least Likely Candidates for Delivery via Needle-Free Injection Systems
- 11.3. Clinical Drug Candidates (Biologics)
- 11.3.1. Most Likely Candidates for Delivery via Needle-Free Injection Systems
- 11.3.2. Likely Candidates for Delivery via Needle-Free Injection Systems
- 11.3.3. Less Likely Candidates for Delivery via Needle-Free Injection Systems
- 11.3.4. Least Likely Candidates for Delivery via Needle-Free Injection Systems
12. COMPANY PROFILES: NEEDLE-FREE DRUG DELIVERY DEVICE PROVIDERS BASED IN NORTH AMERICA
- 12.1. Chapter Overview
- 12.2. Leading Needle-free Drug Delivery Device Providers in North America
- 12.2.1. Antares Pharma (Acquired by Halozyme)
- 12.2.1.1. Company Overview
- 12.2.1.2. Product Portfolio
- 12.2.1.3. Recent Development and Future Outlook
- 12.2.2. D'Antonio Consultants International
- 12.2.3. HNS International
- 12.2.4. INOVIO Pharmaceuticals
- 12.2.5. Medical International Technology
- 12.2.6. PharmaJet
- 12.2.7. Zogenix (acquired by UCB)
- 12.2.1. Antares Pharma (Acquired by Halozyme)
- 12.3. Other Leading Needle-free Drug Delivery Device Providers in North America
- 12.3.1. 3M
- 12.3.1.1. Company Overview
- 12.3.1.2. Product Portfolio
- 12.3.2. TheraJect
- 12.3.1. 3M
13. COMPANY PROFILES: NEEDLE-FREE DRUG DELIVERY DEVICE PROVIDERS BASED IN EUROPE
- 13.1. Chapter Overview
- 13.2. Leading Needle-free Drug Delivery Device Providers in Europe
- 13.2.1. INJEX Pharma
- 13.2.1.1. Company Overview
- 13.2.1.2. Product Portfolio
- 13.2.1.3. Recent Development and Future Outlook
- 13.2.1. INJEX Pharma
- 13.3. Other Leading Needle-free Drug Delivery Device Providers in North America
- 13.3.1. Debiotech
- 13.3.1.1. Company Overview
- 13.3.1.2. Product Portfolio
- 13.3.2. Micropoint Technologies
- 13.3.3. Nemaura Pharma
- 13.3.1. Debiotech
14. COMPANY PROFILES: NEEDLE-FREE DRUG DELIVERY DEVICE PROVIDERS BASED IN ASIA-PACIFIC AND REST OF THE WORLD
- 14.1. Chapter Overview
- 14.2. Leading Needle-free Drug Delivery Device Providers in Asia-Pacific and Rest of the World
- 14.2.1. MIKA MEDICAL
- 14.2.1.1. Company Overview
- 14.2.1.2. Product Portfolio
- 14.2.1.3. Recent Development and Future Outlook
- 14.2.2. QS Medical Technology (Quinovare)
- 14.2.1. MIKA MEDICAL
- 14.3. Other Leading Needle-free Drug Delivery Device Providers in North America
- 14.3.1. INCYTO
- 14.3.1.1. Company Overview
- 14.3.1.2. Product Portfolio
- 14.3.2. NanoPass Technologies
- 14.3.1. INCYTO
15. MEGATRENDS ANALYSIS
- 15.1. Chapter Overview
- 15.2. Key Megatrends
- 15.2.1. Technological Innovations
- 15.2.2. Applications in Personalized Medicine
- 15.2.3. Telemedicine Integration
- 15.2.4. Promising Potential in Driving Sustainability
- 15.2.5. Robust Regulatory Framework
- 15.2.6. Increase in Clinical Trial Activity
16. MARKET IMPACT ANALYSIS
- 16.1. Chapter Overview
- 16.2. Market Drivers
- 16.3. Market Restraints
- 16.4. Market Opportunities
- 16.5. Market Challenges
- 16.6. Conclusion
17. GLOBAL NEEDLE-FREE INJECTION SYSTEMS MARKET
- 17.1. Chapter Overview
- 17.2. Key Assumptions and Methodology
- 17.3. Global Needle-free Injection Systems Market, till 2035
- 17.3.1. Scenario Analysis
- 17.3.1.1. Conservative Scenario
- 17.3.1.2. Optimistic Scenario
- 17.3.1. Scenario Analysis
- 17.4. Key Market Segmentations
18. NEEDLE-FREE INJECTION SYSTEMS MARKET, BY PRODUCT TYPE
- 18.1. Chapter Overview
- 18.2. Key Assumptions and Methodology
- 18.3. Needle-free Injection Systems Market: Distribution by Product Type
- 18.3.1. Needle-free Injection Systems Market for Fillable Devices, till 2035
- 18.3.2. Needle-free Injection Systems Market for Prefilled Devices, till 2035
- 18.4. Data Triangulation and Validation
19. NEEDLE-FREE INJECTION SYSTEMS MARKET, BY ACTUATION MECHANISM
- 19.1. Chapter Overview
- 19.2. Key Assumptions and Methodology
- 19.3. Needle-free Injection Systems Market: Distribution by Actuation Mechanism
- 19.3.1. Needle-free Injection Systems Market for Spring-powered Devices, till 2035
- 19.3.2. Needle-free Injection Systems Market for Compressed-gas Devices, till 2035
- 19.3.3. Needle-free Injection Systems Market for Other Devices, till 2035
- 19.4. Data Triangulation and Validation
20. NEEDLE-FREE INJECTION SYSTEMS MARKET, BY PRODUCT USABILITY
- 20.1. Chapter Overview
- 20.2. Key Assumptions and Methodology
- 20.3. Needle-free Injection Systems Market: Distribution by Product Usability
- 20.3.1. Needle-free Injection Systems Market for Reusable Devices, till 2035
- 20.3.2. Needle-free Injection Systems Market for Disposable Devices, till 2035
- 20.4. Data Triangulation and Validation
21. NEEDLE-FREE INJECTION SYSTEMS MARKET, BY TYPE OF LOAD
- 21.1. Chapter Overview
- 21.2. Key Assumptions and Methodology
- 21.3. Needle-free Injection Systems Market: Distribution by Type of Load
- 21.3.1. Needle-free Injection Systems Market for Liquid-based Devices, till 2035
- 21.3.2. Needle-free Injection Systems Market for Powder-based Devices, till 2035
- 21.3.3. Needle-free Injection Systems Market for Projectile / Depot-based Devices, till 2035
- 21.4. Data Triangulation and Validation
22. NEEDLE-FREE INJECTION SYSTEMS MARKET, BY ROUTE OF ADMINISTRATION
- 22.1. Chapter Overview
- 22.2. Key Assumptions and Methodology
- 22.3. Needle-free Injection Systems Market: Distribution by Route of Administration
- 22.3.1. Needle-free Injection Systems Market for Subcutaneous Route, till 2035
- 22.3.2. Needle-free Injection Systems Market for Intramuscular Route, till 2035
- 22.3.3. Needle-free Injection Systems Market for Intradermal Route, till 2035
- 22.4. Data Triangulation and Validation
23. NEEDLE-FREE INJECTION SYSTEMS MARKET, BY APPLICATION AREA
- 23.1. Chapter Overview
- 23.2. Key Assumptions and Methodology
- 23.3. Needle-free Injection Systems Market: Distribution by Application Area
- 23.3.1. Needle-free Injection Systems Market for Insulin Delivery, till 2035
- 23.3.2. Needle-free Injection Systems Market for Pain Management, till 2035
- 23.3.3. Needle-free Injection Systems Market for Dermatology, till 2035
- 23.3.4. Needle-free Injection Systems Market for Vaccination, till 2035
- 23.4. Data Triangulation and Validation
24. NEEDLE-FREE INJECTION SYSTEMS MARKET, BY GEOGRAPHY
- 24.1. Chapter Overview
- 24.2. Key Assumptions and Methodology
- 24.3. Needle-free Injection Systems Market: Distribution by Geography
- 24.3.1. Needle-free Injection Systems Market in North America, till 2035
- 24.3.1.1. Needle-free Injection Systems Market in the US, till 2035
- 24.3.1.2. Needle-free Injection Systems Market in Canada, till 2035
- 24.3.2. Needle-free Injection Systems Market in Europe, till 2035
- 24.3.2.1. Needle-free Injection Systems Market in Germany, till 2035
- 24.3.2.2. Needle-free Injection Systems Market in the UK, till 2035
- 24.3.2.3. Needle-free Injection Systems Market in France, till 2035
- 24.3.2.4. Needle-free Injection Systems Market in Italy, till 2035
- 24.3.2.5. Needle-free Injection Systems Market in Spain, till 2035
- 24.3.3. Needle-free Injection Systems Market in Asia-Pacific, till 2035
- 24.3.3.1. Needle-free Injection Systems Market in China, till 2035
- 24.3.3.2. Needle-free Injection Systems Market in India, till 2035
- 24.3.3.3. Needle-free Injection Systems Market in Japan, till 2035
- 24.3.3.4. Needle-free Injection Systems Market in Australia, till 2035
- 24.3.4. Needle-free Injection Systems Market in Middle East and North Africa, till 2035
- 24.3.4.1. Needle-free Injection Systems Market in Egypt, till 2035
- 24.3.4.2. Needle-free Injection Systems Market in Saudi Arabia, till 2035
- 24.3.4.3. Needle-free Injection Systems Market in Israel, till 2035
- 24.3.5. Needle-free Injection Systems Market in Latin America, till 2035
- 24.3.5.1. Needle-free Injection Systems Market in Brazil, till 2035
- 24.3.5.2. Needle-free Injection Systems Market in Mexico, till 2035
- 24.3.5.3. Needle-free Injection Systems Market in Argentina, till 2035
- 24.3.1. Needle-free Injection Systems Market in North America, till 2035
- 24.4. Data Triangulation and Validation
25. GLOBAL MICRONEEDLE DEVICES MARKET
- 25.1. Chapter Overview
- 25.2. Key Assumptions and Methodology
- 25.3. Global Microneedle Devices Market, till 2035
- 25.3.1. Scenario Analysis
- 25.3.1.1. Conservative Scenario
- 25.3.1.2. Optimistic Scenario
- 25.3.1. Scenario Analysis
- 25.4. Key Market Segmentations
26. MICRONEEDLE DEVICES MARKET, BY TYPE OF MICRONEEDLE
- 26.1. Chapter Overview
- 26.2. Key Assumptions and Methodology
- 26.3. Microneedle Devices Market: Distribution by Type of Microneedle
- 26.3.1. Microneedle Devices Market for Hollow Microneedles, till 2035
- 26.3.2. Microneedle Devices Market for Solid Microneedles, till 2035
- 26.3.3. Microneedle Devices Market for Dissolving Microneedles, till 2035
- 26.3.4. Microneedle Devices Market for Coated Microneedles, till 2035
- 26.4. Data Triangulation and Validation
27. MICRONEEDLE DEVICES MARKET, BY TYPE OF MATERIAL USED
- 27.1. Chapter Overview
- 27.2. Key Assumptions and Methodology
- 27.3. Microneedle Devices Market: Distribution by Type of Material Used
- 27.3.1. Microneedle Devices Market for Polymer Microneedles, till 2035
- 27.3.2. Microneedle Devices Market for Silicon Microneedles, till 2035
- 27.3.3. Microneedle Devices Market for Metal Microneedles, till 2035
- 27.3.4. Microneedle Devices Market for Ceramic Microneedles, till 2035
- 27.4. Data Triangulation and Validation
28. MICRONEEDLE DEVICES MARKET, BY APPLICATION AREA
- 28.1. Chapter Overview
- 28.2. Key Assumptions and Methodology
- 28.3. Microneedle Devices Market: Distribution by Application Area
- 28.3.1. Microneedle Devices Market for Insulin Delivery, till 2035
- 28.3.2. Microneedle Devices Market for Pain Management, till 2035
- 28.3.3. Microneedle Devices Market for Vaccination, till 2035
- 28.3.4. Microneedle Devices Market for Oncology, till 2035
- 28.4. Data Triangulation and Validation
29. MICRONEEDLE DEVICES MARKET, BY TYPE OF INTERVENTION
- 29.1. Chapter Overview
- 29.2. Key Assumptions and Methodology
- 29.3. Microneedle Devices Market: Distribution by Type of Intervention
- 29.3.1. Microneedle Devices Market for Vaccines, till 2035
- 29.3.2. Microneedle Devices Market for Therapeutic Agents, till 2035
- 29.3.3. Microneedle Devices Market for Other Interventions, till 2035
- 29.4. Data Triangulation and Validation
30. MICRONEEDLE DEVICES MARKET, BY GEOGRAPHY
- 30.1. Chapter Overview
- 30.2. Key Assumptions and Methodology
- 30.3. Microneedle Devices Market: Distribution by Geography
- 30.3.1. Microneedle Devices Market in North America, till 2035
- 30.3.1.1. Microneedle Devices Market in the US, till 2035
- 30.3.1.2. Microneedle Devices Market in Canada, till 2035
- 30.3.2. Microneedle Devices Market in Europe, till 2035
- 30.3.2.1. Microneedle Devices Market in France, till 2035
- 30.3.2.2. Microneedle Devices Market in Spain, till 2035
- 30.3.2.3. Microneedle Devices Market in the UK, till 2035
- 30.3.2.4. Microneedle Devices Market in Germany, till 2035
- 30.3.2.5. Microneedle Devices Market in Italy, till 2035
- 30.3.3. Microneedle Devices Market in Asia-Pacific, till 2035
- 30.3.3.1. Microneedle Devices Market in China, till 2035
- 30.3.3.2. Microneedle Devices Market in Japan, till 2035
- 30.3.3.3. Microneedle Devices Market in India, till 2035
- 30.3.3.4. Microneedle Devices Market in Australia, till 2035
- 30.3.4. Microneedle Devices Market in Middle East and North Africa, till 2035
- 30.3.4.1. Microneedle Devices Market in Egypt, till 2035
- 30.3.4.2. Microneedle Devices Market in Saudi Arabia, till 2035
- 30.3.4.3. Microneedle Devices Market in Israel, till 2035
- 30.3.5. Microneedle Devices Market in Latin America, till 2035
- 30.3.5.1. Microneedle Devices Market in Brazil, till 2035
- 30.3.5.2. Microneedle Devices Market in Mexico, till 2035
- 30.3.5.3. Microneedle Devices Market in Argentina, till 2035
- 30.3.1. Microneedle Devices Market in North America, till 2035
- 30.4. Data Triangulation and Validation
31. CONCLUDING REMARKS
32. EXECUTIVE INSIGHTS
- 32.1. Chapter Overview
- 32.2. Portal Instruments
- 32.2.1. Company Snapshot
- 32.2.2. Interview Transcript
- 32.3. Vaxess Technologies
- 32.3.1 Company Snapshot
- 32.3.2. Interview Transcript
- 32.4. Innoture
- 32.4.1. Company Snapshot
- 32.4.2. Interview Transcript