
|
시장보고서
상품코드
1682705
경구 고형 제제 제조 시장 : 완성 제형별, 포장 형태별, 사업 규모별, 기업 규모별, 치료 영역별, 주요 지역별 - 업계 동향과 세계 예측(- 2035년)Oral Solid Dosage Manufacturing Market by Type of Finished Dosage Form, Type of Packaging, Scale of Operation, Company Size, Therapeutic Area, and Key Geographical Regions : Industry Trends and Global Forecasts, Till 2035 |
||||||
세계 경구 고형 제제 제조 시장 규모는 2035년까지 예측 기간 동안 4.49%의 연평균 복합 성장률(CAGR)로 확대되어 현재 225억 달러에서 2035년까지 365억 2,000만 달러로 성장할 것으로 예상됩니다.
의약품 및 건강기능식품 분야에서 여러 가지 새로운 제형이 도입되고 있음에도 불구하고, 경구용 고형제(OSD), 특히 정제 및 캡슐은 여전히 가장 인기 있고 널리 사용되는 전달 형태입니다. 이는 이러한 제형이 자가 투여, 안정성, 비용 효율성, 취급 및 운송 편의성, 환자 순응도 등 다양한 이점을 제공하기 때문입니다. 제약 산업 전체에서 이 성숙하고도 고부가가치 분야는 인체용 제제 세계 시장 점유율의 약 90%를 차지하고 있습니다.
그러나 OSD 제조 공정은 특수 장비와 시설, 고도로 통제된 작업 환경, 다학제적 지식을 갖춘 전문가를 필요로 하는 복잡한 과정입니다. 또한, 낮은 생체 이용률, 낮은 용해도, 쓴맛, 높은 역가 등 원료의약품과 관련된 업스트림 병목현상을 해결하기 위한 최근의 발전은 광범위한 기술적 전문지식을 필요로 합니다. 그 결과, 제약사들은 비용 절감 기회를 얻기 위해 위탁 서비스 제공업체에 대한 의존도를 높이고 있습니다. 제약회사와 달리, 이러한 제3자 서비스 제공업체의 역량은 일반적으로 각각의 서비스 포트폴리오에 집중되어 있습니다. 제약사들은 제법 및 제제 개발부터 원료의약품 특성 평가, 분석법 개발, 규제 당국 신청, 임상 및 상업 생산 능력에 이르기까지 전문적인 역량으로 제약사를 지원하고 있습니다. 아웃소싱의 확대 추세와 서비스 제공업체들이 지속적으로 서비스 개선 및 확대에 힘쓰고 있는 점을 고려할 때, 경구용 고형제 위탁생산 시장은 중장기적으로 꾸준한 성장세를 보일 것으로 예상됩니다.
현재 300개 이상의 기업이 정제 및 캡슐 서방형 제제를 포함한 광범위한 경구용 고형제 수탁 제조 서비스를 제공하는 데 필요한 전문 지식을 보유하고 있다고 주장하고 있습니다. 약 30%의 기업이 초기 개발부터 다양한 용기에 경구용 고형 제제의 포장에 이르기까지 엔드 투 엔드 역량을 갖추고 있어 스폰서 기업공급업체 관리 활동을 간소화할 수 있습니다. 현재 시장 상황은 규제 상황에 따라 다양한 규모의 경구 고형 제제 제조에 종사하는 기존 및 신규 시장 진출기업들이 존재하며, 1,350개 이상의 시설을 갖춘 경구용 고형제 위탁 생산 업체들이 전 세계적으로 존재감을 드러내고 있습니다. 이들 업체 대부분은 주로 중국, 인도 등 아시아태평양에 기반을 두고 있습니다.
세계의 경구 고형 제제 제조 시장에 대해 조사했으며, 시장 개요와 함께 완제 형태별/포장 형태별/사업 규모별/기업 규모별/치료 영역별/주요 지역별 동향, 시장 진출기업 프로파일 등의 정보를 전해드립니다.
목차
제1장 서문
제2장 주요 요약
제3장 서론
제4장 경구 고형 제제 수탁제조조직(CMO) : 시장 구도
제5장 기업 경쟁력 분석
제6장 지역 능력 분석
제7장 기업 개요
제8장 제조 vs. 구입 의사 의사결정 프레임워크
제9장 최근 확장
제10장 용량 분석
제11장 수요 분석
제12장 경구 고형 제제 수탁제조조직의 총 소유비용
제13장 경구 고형 제제 계약 제조업체에 대한 규제 상황
제14장 미각 마스킹 서비스와 기술에 관한 사례 연구 : 시장 구도
제15장 생체이용률 향상 기술에 관한 사례 연구 : 시장 구도
제16장 시장 예측
제17장 주요 인사이트
제18장 결론
제19장 부록 1 : 표 형식 데이터
제20장 부록 2 : 기업 및 단체 리스트
LSH 25.03.27ORAL SOLID DOSAGE CONTRACT MANUFACTURING MARKET: OVERVIEW
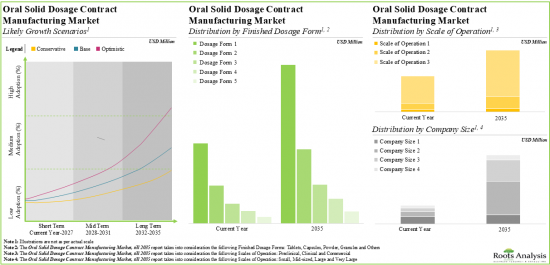
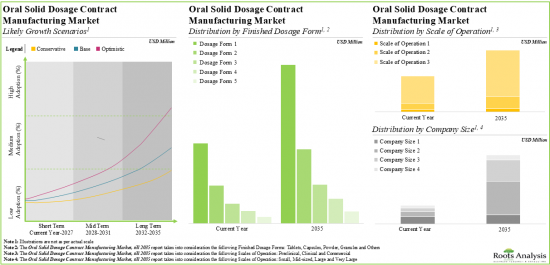
As per Roots Analysis, the global oral solid dosage contract manufacturing market is estimated to grow from USD 22.5 billion in the current year to USD 36.52 billion by 2035, at a CAGR of 4.49% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Finished Dosage Form
- Tablets
- Capsules
- Powders
- Multi-particulates
- Others
Type of Packaging
- Bottles
- Blisters
- Sachets
- Inhalers
- Others
Scale of Operation
- Pre-commercial
- Commercial
Company Size
- Small
- Mid-sized
- Large
- Very Large
Therapeutic Area
- Oncological Disorders
- Infectious Diseases
- Cardiovascular Disorders
- Metabolic Disorders
- Neurological Disorders
- Genetic Disorders
- Respiratory Disorders
- Immunological Disorders
- Other Disorders
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and North Africa
ORAL SOLID DOSAGE CONTRACT MANUFACTURING MARKET: GROWTH AND TRENDS
Despite several novel dosage forms being introduced into the pharmaceutical and nutraceutical sectors, oral solid drugs (OSD), particularly tablets and capsules, continue to be the most popular and widely used delivery forms. This is due to the fact that these formulations offer various advantages, including self-administration, stability, cost-effectiveness, convenience of handling, transportation and patient compliance. This mature yet high-value vertical of the overall pharmaceutical industry represents about 90% of the global market share of all formulations intended for human use.
However, OSD manufacturing operations is a complex process requiring specialized equipment and facilities, highly contained working environments and experts with multidisciplinary knowledge. Further, recent evolution to address the upstream bottlenecks associated with active pharmaceutical ingredients (API), such as poor bioavailability, low solubility, bitter taste and high potency, necessitate extensive technical expertise. As a result, drug developers are increasingly relying on contract service providers to leverage their capabilities and yield cost savings opportunities. Unlike drug developers, the capabilities of these third-party service providers are usually more focused on their respective service portfolios. They support pharmaceutical companies with specialized capabilities ranging from process and formulation development to drug substance characterization, analytical method development, and regulatory filings, as well as capacity for clinical and commercial manufacturing. Considering the growing trend of outsourcing and the ongoing efforts of service providers to improve / expand their offerings, it is anticipated that the oral solid dosage contract manufacturing market is likely to evolve at a steady pace, in the mid to long term.
ORAL SOLID DOSAGE CONTRACT MANUFACTURING MARKET: KEY INSIGHTS
The report delves into the current state of the oral solid dosage contract manufacturing market and identifies potential growth opportunities within the industry. Some key findings from the report include:
1. Presently, over 300 companies claim to have the required expertise to offer contract manufacturing services for a broad spectrum of oral solid dosage forms, including modified release formulations of tablets and capsules.
2. Nearly 30% of the players have established end-to-end capabilities, from early development to packaging of oral solids in a variety of containers; this simplifies supplier management activities for sponsor companies.
3. The current market landscape features the presence of both established and emerging players engaged in the manufacturing of oral solid dosages at various scales of operation, in compliance with the regulatory standards.
4. With more than 1,350 facilities, oral solid contract manufacturers have established global presence; majority of these players are based in the Asia-Pacific region, primarily in countries, such as China and India.
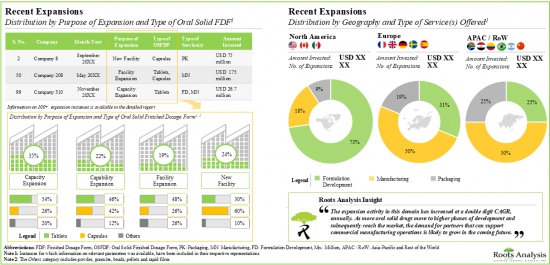
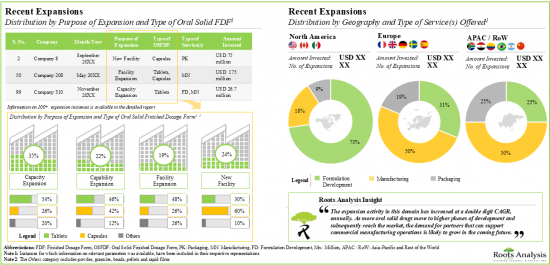
5. As part of their growth strategy, CMOs are investing in either expanding their existing facilities / capabilities or building facilities in other regions; majority of these initiatives were undertaken in North America.
6. The global installed oral solid dosage contract manufacturing capacity is spread across various geographies; over 30% of this capacity is dedicated to the manufacturing of tablets on a commercial scale.
7. The demand for oral solid dosage contract manufacturing is rising significantly owing to the growing complexity of APIs; by 2035, it is anticipated to reach over 35,000 metric tonnes, across clinical and commercial scales.
8. Driven by volume shifts from originators to generics and over-investments to create safety buffers, the contract manufacturing domain is currently witnessing an excess in capacity, thereby propelling consolidation efforts.
9. Over 65% of the market is expected to be captured by revenues from manufacturing anti-cancer drugs, including those based on HPAPIs; furthermore, North America based players are likely to contribute majorly to this domain.
ORAL SOLID DOSAGE CONTRACT MANUFACTURING MARKET: KEY SEGMENTS
Tablets Occupy the Largest Share of the Oral Solid Dosage Contract Manufacturing Market
Based on the type of finished dosage form, the market is segmented into tablets, capsules, powders, multi-particulates and others. At present, tablets hold the maximum share of the oral solid dosage contract manufacturing market. This trend is likely to remain the same in the forthcoming years.
Blisters Segment is the Fastest Growing Segment of the Oral Solid Dosage Contract Manufacturing Market During the Forecast Period
Based on the type of packaging, the market is segmented into blisters, sachets, inhalers, bottles and others. Currently, bottles capture the highest proportion of the oral solid dosage contract manufacturing market. It is worth highlighting that the oral solid dosage contract manufacturing market for blisters is likely to grow at a relatively higher CAGR.
By Scale of Operation, Commercial Scale is Likely to Dominate the Oral Solid Dosage Contract Manufacturing Market
Based on the scales of operation, the market is segmented into pre-commercial and commercial scale. Whilst commercial scale manufacturing is expected to be the primary driver of the overall market, it is worth highlighting that the oral solid dosage contract manufacturing market at pre-commercial scale is likely to grow at a relatively higher CAGR.
Very Large Companies Occupy the Largest Share of the Oral Solid Dosage Contract Manufacturing Market
Based on the company size, the market is segmented into small, mid-sized, large and very large companies. At present, very large companies hold the maximum share of the oral solid dosage contract manufacturing market. This trend is unlikely to change in the foreseeable future.
Oncological Disorders Account for the Largest Share of the Oral Solid Dosage Contract Manufacturing Market
Based on the therapeutic areas, the market is segmented into oncological disorders, neurological disorders, cardiovascular disorders, infectious diseases, metabolic disorders, respiratory disorders, immunological disorders, genetic disorders, gastrointestinal disorders, other disorders. Currently, oncological disorders hold the maximum share of the oral solid dosage contract manufacturing market. This trend is likely to remain the same in the coming decade.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific, Latin America, and Middle East and North Africa. The majority share is expected to be captured by players based in North America and Asia-Pacific.
Example Players in the Oral Solid Dosage Contract Manufacturing Market
- Aenova
- Alcami
- Almac
- Cambrex
- Hetero Drugs
- Ind-Swift
- Laboratories
- Lonza
- Rubicon Research
ORAL SOLID DOSAGE CONTRACT MANUFACTURING MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the oral solid dosage contract manufacturing market, focusing on key market segments, including [A] type of finished dosage form, [B] type of packaging, [C] scale of operation, [D] company size, [E] therapeutic area and [F] key geographical regions.
- Market Landscape: A comprehensive evaluation of companies offering contract services for oral solid dosage form, considering various parameters, such as [A] year of establishment, [B] company size (in terms of the number of employees), [C] location of headquarters, [D] company ownership, [E] type of service(s) offered, [F] type of finished dosage form(s) manufactured, [G] type of packaging offered, [H] scale(s) of operation and [I] additional manufacturing capabilities.
- Company Competitiveness Analysis: A comprehensive competitive analysis of oral solid dosage contract manufacturing service providers, examining factors, such as [A] supplier strength and [B] service strength.
- Regional Capability Analysis: A comprehensive regional capability assessment framework the oral solid dosage contract manufacturers that evaluates key geographies by various parameters, including [A] type of service(s) offered, [B] type of finished dosage form(s) manufactured, [C] type of packaging offered, [D] scale(s) of operation and [E] location of manufacturing facilities of the service providers.
- Company Profiles: In-depth profiles of key oral solid dosage contract manufacturers, focusing on [A] company overviews, [B] financial information (if available), [C] oral solid dosage service portfolio, [D] manufacturing capabilities and facilities, [E] recent developments and [F] an informed future outlook.
- Make Versus Buy Decision Framework: An insightful framework that emphasizes the key indicators and factors that need to be considered by oral solid dosage drug developers to determine whether to manufacture their respective products in-house or outsource the manufacturing operation to contract service providers.
- Recent Expansions: An examination of the different expansion efforts made by service providers in this field to enhance their manufacturing capabilities. This analysis considers various factors, including the [A] year of expansion, [B] purpose of expansion, [C] type of an estimate of the global installed capacity (in terms of number of oral solid units and volume of API) for the manufacturing of oral solids, based on information provided by various industry stakeholders in the public domain. It also features the distribution of the available capacity on the basis of [D] company size, [E] scale of operation, [F] type of finished dosage form and [G] key geographical regions. Overall, the analysis represents a holistic view of the supply-side of the market, allowing us to present an informed opinion on whether the present capacity will be able to meet the likely future demand.
- Demand Analysis: Informed estimates of the annual commercial and clinical demand for oral solid doses based on several relevant parameters, such as [A] key geographical regions and [B] therapeutic areas.
- Total Cost of Ownership: A detailed analysis of the total cost of ownership for an oral solid dosage contract manufacturing service provider, highlighting the expenses associated with the establishment and maintenance of an oral solid dosage manufacturing facility, over a period of 20 years.
- Regulatory Landscape: A discussion on general regulatory guidelines laid down by major regulatory bodies, across different countries, featuring an elaborative assessment of several CMOs engaged in this domain, along with information on their operational approvals, certifications received, and relative popularity of the key regulatory body. Additionally, the chapter features an insightful multi-dimensional bubble analysis, presenting a comparison of the current regulatory scenario in key geographies.
- Case Study 1: A general discussion on taste masking service providers for oral solids, providing information on [A] scale of operation, [B] manufacturing scalability, other service(s) offered along with information on [C] type of formulation, [D] end users, [E] techniques used for taste masking, [F] branch of healthcare and [G] regional capability. Further, the chapter includes the current market landscape of taste masking technology providers, providing information on [H] technology name, [I] techniques used, [J] type of finished dosage form, [K] end users along with information on patent, availability of technology for partnerships and licensing. In addition, the chapter provides details on the [L] year of establishment, [M] company size and [N] location of headquarters.
- Case Study 2: A general discussion on drug bioavailability enhancement service providers for oral solids, based on several relevant parameters, such as [A] year of establishment, [B] company size (in terms of number of employees), [C] location of headquarters, [D] bioavailability enhancement principle supported, [E] bioavailability enhancement approach employed, including [E1] solid dispersion, [E2] size reduction, [E3] lipid-based, and [E4] other bioavailability enhancement approaches, [F] type of dosage form supported (solids, fine particles and semi-solids).
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 10% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Key Market Insights
- 1.3. Scope of the Report
- 1.4. Research Methodology
- 1.5. Frequently Asked Questions
- 1.6. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Type of Dosage Forms
- 3.3. Overview of Oral Solid Dosage Forms
- 3.3.1. Components of Oral Solid Dosage Forms
- 3.3.2. Classification of Oral Solid Dosage Forms
- 3.3.3. Manufacturing of Oral Solid Dosage Forms
- 3.3.4. Emerging Trends in Oral Solid Dosage Manufacturing
- 3.4. Overview of Oral Solid Contract Manufacturing
- 3.4.1. Services Offered by CMOs and CDMOs for Oral Solid Manufacturing
- 3.4.2. Key Considerations While Selecting a CDMO or CMO Partner
- 3.4.3.Risks and Challenges Associated with Outsourcing Pharmaceutical Manufacturing Operations
- 3.5. Future Perspectives
4. ORAL SOLID DOSAGE CONTRACT MANUFACTURING ORGANIZATIONS (CMOs): MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Oral Solid Dosage CMOs: List of Companies
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Location of Headquarters
- 4.2.4. Analysis by Company Ownership
- 4.2.5. Analysis by Type of Service(s) Offered
- 4.2.6. Analysis by Type of Finished Dosage Form
- 4.2.7. Analysis by Type of Tablet(S) Manufactured
- 4.2.8. Analysis by Type of Capsule(s) Manufactured
- 4.2.9. Analysis by Type of Multi-particulate(s) Manufactured
- 4.2.10. Analysis by Type of Primary Packaging Offered
- 4.2.11. Analysis by Scale of Operation
- 4.2.12. Analysis by High Potency Drug Manufacturing
- 4.2.13. Analysis by Availability of Continuous Manufacturing
- 4.2.14. Analysis by Regulatory Certifications / Accreditations
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Assumptions and Key Parameters
- 5.3. Methodology
- 5.4. Company Competitiveness Analysis: Oral Solid Dosage Contract Manufacturers in North America
- 5.4.1. Peer Group I: Companies in the US
- 5.4.2. Peer Group II: Companies in Canada
- 5.5. Company Competitiveness Analysis: Oral Solid Dosage Contract Manufacturers based in Europe
- 5.5.1. Peer Group III: Companies in EU5
- 5.5.2. Peer Group IV: Other European Countries
- 5.6. Company Competitiveness Analysis: Oral Solid Contract Manufacturers based in Asia-Pacific and Rest of the World
- 5.6.1. Peer Group V: Companies in India
- 5.6.2. Peer Group VI: Companies in China
- 5.6.3. Peer Group VII: Companies in Japan
- 5.6.4. Peer Group VIII: Companies in Other Asia-Pacific and rest of the World
- 5.7. Company Competitiveness Analysis of Oral Solid Dosage Contract Manufacturers: Competitiveness Score of Top Service Providers
6. REGIONAL CAPABILITY ANALYSIS
- 6.1. Chapter Overview
- 6.2. Key Assumptions and Methodology
- 6.3. Overall Landscape of Oral Solid Dosage Contract Manufacturing Facilities
- 6.4. Oral Solid Dosage Contract Manufacturers in North America
- 6.5. Oral Solid Dosage Contract Manufacturers in Europe
- 6.6. Oral Solid Dosage Contract Manufacturers in Asia-Pacific and Rest of the World
7. COMPANY PROFILES
- 7.1. Chapter Overview
- 7.2. Oral Solid Dosage Contract Manufacturing Service Providers: North America
- 7.2.1. Alcami
- 7.2.1.1. Company Overview
- 7.2.1.2. Service Portfolio
- 7.2.1.2.1. Manufacturing Capabilities and Facilities
- 7.2.1.3. Recent Developments and Future Outlook
- 7.2.2. Cambrex
- 7.2.2.1. Company Overview
- 7.2.2.2. Service Portfolio
- 7.2.2.2.1. Manufacturing Capabilities and Facilities
- 7.2.2.3. Recent Developments and Future Outlook
- 7.2.3. Catalent
- 7.2.3.1. Company Overview
- 7.2.3.2. Financial Information
- 7.2.3.3. Service Portfolio
- 7.2.3.3.1. Manufacturing Capabilities and Facilities
- 7.2.3.4. Recent Developments and Future Outlook
- 7.2.1. Alcami
- 7.3. Oral Solid Dosage Contract Manufacturing Service Providers: Europe
- 7.3.1. Aenova
- 7.3.1.1. Company Overview
- 7.3.1.2. Financial Information
- 7.3.1.3. Service Portfolio
- 7.3.1.3.1. Manufacturing Capabilities and Facilities
- 7.3.1.4. Recent Developments and Future Outlook
- 7.3.2. Almac
- 7.3.2.1. Company Overview
- 7.3.2.2. Financial Information
- 7.3.2.3. Service Portfolio
- 7.3.2.3.1. Manufacturing Capabilities and Facilities
- 7.3.2.4. Recent Developments and Future Outlook
- 7.3.3. Lonza
- 7.3.3.1. Company Overview
- 7.3.3.2. Financial Information
- 7.3.3.3. Service Portfolio
- 7.3.3.3.1. Manufacturing Capabilities and Facilities
- 7.3.3.4. Recent Developments and Future Outlook
- 7.3.1. Aenova
- 7.4. Oral Solid Dosage Contract Manufacturing Service Providers: Asia-Pacific and Rest of the World
- 7.4.1. Hetero Drugs
- 7.4.1.1. Company Overview
- 7.4.1.2. Service Portfolio
- 7.4.1.2.1. Manufacturing Capabilities and Facilities
- 7.4.1.3. Recent Developments and Future Outlook
- 7.4.2. Ind-Swift Laboratories
- 7.4.2.1. Company Overview
- 7.4.2.2. Financial Information
- 7.4.2.3. Service Portfolio
- 7.4.2.3.1. Manufacturing Capabilities and Facilities
- 7.4.2.4. Recent Developments and Future Outlook
- 7.4.3. Rubicon Research
- 7.4.3.1. Company Overview
- 7.4.3.2. Service Portfolio
- 7.4.3.2.1. Manufacturing Capabilities and Facilities
- 7.4.3.3. Recent Developments and Future Outlook
- 7.4.1. Hetero Drugs
8. MAKE VERSUS BUY DECISION FRAMEWORK
- 8.1. Chapter Overview
- 8.2. Assumptions and Key Parameters
- 8.3. Oral Solid Dosage Manufacturers: Make versus Buy Decision Making
- 8.3.1. Scenario 1
- 8.3.2. Scenario 2
- 8.3.3. Scenario 3
- 8.3.4. Scenario 4
- 8.4. Concluding Remarks
9. RECENT EXPANSIONS
- 9.1. Chapter Overview
- 9.2. Oral Solid Dosage Contract Manufacturers: Recent Expansions
- 9.2.1. Analysis by Year of Expansion
- 9.2.2. Analysis by Type of Expansion
- 9.2.3. Analysis by Year and Type of Expansion
- 9.2.4. Analysis by Type of Oral Solid Dosage Form(s) Involved
- 9.2.5. Analysis by Type of Expansion and Oral Solid Dosage Form(s) Involved
- 9.2.6. Analysis by Type of Service(s) Offered
- 9.2.7. Analysis by Scales of Operation
- 9.2.8. Analysis by Location of Facility
- 9.2.9. Expansions Focused on Highly Potent Compounds
10. CAPACITY ANALYSIS
- 10.1. Chapter Overview
- 10.2. Key Assumptions and Methodology
- 10.2.1. Oral Solid Dosage Contract Manufacturing: Annual Global Capacity (Number of Finished Dosages)
- 10.2.1.1. Analysis by Company Size
- 10.2.1.2. Analysis by Scale of Operation
- 10.2.1.3. Analysis by Type of Finished Dosage Form
- 10.2.1.4. Analysis by Location of Manufacturing Facility
- 10.2.1.5. Analysis by Company Size and Type of Finished Dosage Form
- 10.2.1.6. Analysis by Company Size and Location of Manufacturing Facility
- 10.2.1.7. Analysis by Scale of Operation and Location of Manufacturing Facility
- 10.2.2. Oral Solid Dosage Contract Manufacturing: Annual Global Capacity (Amount of API)
- 10.2.2.1. Analysis by Company Size
- 10.2.2.2. Analysis by Scale of Operation
- 10.2.2.3. Analysis by Type of Finished Dosage Form
- 10.2.2.4. Analysis by Company Size and Type of Finished Dosage Form
- 10.2.2.5. Analysis by Company Size and Location of Manufacturing Facility
- 10.2.2.6. Analysis by Scale of Operation and Location of Manufacturing Facility
- 10.2.1. Oral Solid Dosage Contract Manufacturing: Annual Global Capacity (Number of Finished Dosages)
11. DEMAND ANALYSIS
- 11.1. Chapter Overview
- 11.2. Key Assumptions and Methodology
- 11.3. Overall Annual Demand for Oral Solids, till 2035
- 11.4. Annual Outsourced Commercial Demand for Oral Solids, till 2035
- 11.4.1. Annual Outsourced Commercial Demand for Oral Solids: Distribution by Geography, Current Year and 2035
- 11.4.1.1. Annual Outsourced Commercial Demand for Oral Solids in North America, till 2035
- 11.4.1.2. Annual Outsourced Commercial Demand for Oral Solids in Europe, till 2035
- 11.4.1.3. Annual Outsourced Commercial Demand for Oral Solids in Asia-Pacific and Rest of the World, till 2035
- 11.4.2. Annual Outsourced Commercial Demand for Oral Solids: Distribution by Therapeutic Area, Current Year and 2035
- 11.4.2.1. Annual Outsourced Commercial Demand for Oral Solids for Oncological Disorders, till 2035
- 11.4.2.2. Annual Outsourced Commercial Demand for Oral Solids for Infectious Diseases, till 2035
- 11.4.2.3. Annual Outsourced Commercial Demand for Oral Solids for Cardiovascular Disorders, till 2035
- 11.4.2.4. Annual Outsourced Commercial Demand for Oral Solids for Metabolic Disorders, till 2035
- 11.4.2.5. Annual Outsourced Commercial Demand for Oral Solids for Neurological Disorders, till 2035
- 11.4.2.6. Annual Outsourced Commercial Demand for Oral Solids for Genetic Disorders, till 2035
- 11.4.2.7. Annual Outsourced Commercial Demand for Oral Solids for Respiratory Disorders, till 2035
- 11.4.2.8. Annual Outsourced Commercial Demand for Oral Solids for Immunological Disorders, till 2035
- 11.4.2.9. Annual Outsourced Commercial Demand for Oral Solids for Other Disorders, till 2035
- 11.4.1. Annual Outsourced Commercial Demand for Oral Solids: Distribution by Geography, Current Year and 2035
- 11.5. Annual Outsourced Clinical Demand for Oral Solids, till 2035
- 11.5.1. Annual Outsourced Clinical Demand for Oral Solids: Distribution by Trial Phase, till 2035
- 11.5.1.1. Annual Outsourced Clinical Demand for Oral Solids in Phase I Trials, till 2035
- 11.5.1.2. Annual Outsourced Clinical Demand for Oral Solids in Phase II Trials, till 2035
- 11.5.1.3. Annual Outsourced Clinical Demand for Oral Solids in Phase III Trials, till 2035
- 11.5.1. Annual Outsourced Clinical Demand for Oral Solids: Distribution by Trial Phase, till 2035
- 11.6. Correlation between Annual Demand and Capacity
12. TOTAL COST OF OWNERSHIP FOR ORAL SOLID DOSAGE CONTRACT MANUFACTURING ORGANIZATIONS
- 12.1. Chapter Overview
- 12.2. Key Parameters
- 12.3. Assumptions and Methodology
- 12.4. Sample Dataset for the Estimation of Total Cost of Ownership
- 12.5. Total Cost of Ownership for Oral Solid Dosage Contract Manufacturing Organizations, Y0-Y20
- 12.6. Total Cost of Ownership for Oral Solid Dosage Contract Manufacturing Organizations: Analysis by CapEx and OpEx, Y0 and Y20
- 12.6.1. Total Cost of Ownership for Oral Solid Contract Manufacturing Organizations: Analysis by CapEx, Y0
- 12.6.2. Total Cost of Ownership for Oral Solid Dosage Contract Manufacturing Organizations: Analysis by OpEx, Y0-Y20
- 12.7. Concluding Remarks
13. REGULATORY LANDSCAPE FOR ORAL SOLID DOSAGE CONTRACT MANUFACTURERS
- 13.1. Chapter Overview
- 13.2. Regulatory Guidelines in North America
- 13.2.1. The US Scenario
- 13.2.1. Canadian Scenario
- 13.3. Regulatory Guidelines in Europe
- 13.4. Regulatory Guidelines in Asia-Pacific and Rest of the World
- 13.4.1. Chinese Scenario
- 13.4.2. Indian Scenario
- 13.4.3. Japanese Scenario
- 13.4.4. South Korean Scenario
- 13.4.5. Australian Scenario
- 13.4.6. Brazilian Scenario
- 13.5. Oral Solid Dosage CMOs: Information on Approval From Various Regulatory Authorities
- 13.6. Bubble Analysis: Regional Regulatory Summary
14. CASE STUDY ON TASTE MASKING SERVICES AND TECHNOLOGIES: MARKET LANDSCAPE
- 14.1. Chapter Overview
- 14.2. Taste Masking and Taste Assessment Service Providers
- 14.2.1. Analysis by Year of Establishment
- 14.2.2. Analysis by Company Size
- 14.2.3. Analysis by Location of Headquarters
- 14.2.4. Analysis by Year of Establishment and Location of Headquarters
- 14.3. Taste Masking and Taste Assessment Services: Overall Market Landscape
- 14.3.1. Analysis by Service(s) Offered
- 14.3.2. Analysis by Scale of Operation
- 14.3.3. Analysis by Other Service(s) Offered
- 14.3.4. Analysis by Type of Formulation(s)
- 14.3.5. Analysis by End Users
- 14.3.6. Analysis by Techniques Used for Taste Masking
- 14.3.7. Analysis by Branch of Healthcare
- 14.3.8. Analysis by Regional Capability
15. CASE STUDY ON BIOAVAILABILITY ENHANCEMENT TECHNOLOGIES: MARKET LANDSCAPE
- 15.1. Chapter Overview
- 15.2. Bioavailability Enhancement Service Providers: Overall Market Landscape
- 15.2.1. Analysis by Year of Establishment
- 15.2.2. Analysis by Company Size
- 15.2.3. Analysis by Location of Headquarters
- 15.2.4. Analysis by Bioavailability Enhancement Principle
- 15.2.5. Analysis by Bioavailability Enhancement Approach
- 15.2.5.1. Analysis by Solid Dispersion Approaches
- 15.2.5.2. Analysis by Size Reduction Approaches
- 15.2.5.3. Analysis by Lipid-Based Approaches
- 15.2.5.4. Analysis by Other Bioavailability Enhancement Approaches
- 15.2.6. Analysis by Dosage Form
- 15.2.7. Analysis by Route of Administration
16. MARKET FORECAST
- 16.1. Chapter Overview
- 16.2. Forecast Methodology and Key Assumptions
- 16.2.1. Overall Oral Solid Dosage Contract Manufacturing Market, till 2035
- 16.2.2. Oral Solid Dosage Contract Manufacturing Market, till 2035: Distribution by Type of Finished Dosage Form
- 16.2.2.1. Oral Solid Dosage Contract Manufacturing Market for Tablets, till 2035
- 16.2.2.2. Oral Solid Dosage Contract Manufacturing Market for Capsules, till 2035
- 16.2.2.3. Oral Solid Dosage Contract Manufacturing Market for Powders, till 2035
- 16.2.2.4. Oral Solid Dosage Contract Manufacturing Market for Multi-particulates, till 2035
- 16.2.2.5. Oral Solid Dosage Contract Manufacturing Market for Other Finished Dosage Forms, till 2035
- 16.2.3. Oral Solid Dosage Contract Manufacturing Market, till 2035: Distribution by Type of Packaging
- 16.2.3.1. Oral Solid Dosage Contract Manufacturing Market for Bottles, till 2035
- 16.2.3.2. Oral Solid Dosage Contract Manufacturing Market for Blisters, till 2035
- 16.2.3.3. Oral Solid Dosage Contract Manufacturing Market for Inhalers, till 2035
- 16.2.3.4. Oral Solid Dosage Contract Manufacturing Market for Sachets, till 2035
- 16.2.3.5. Oral Solid Dosage Contract Manufacturing Market for Strips, till 2035
- 16.2.3.6. Oral Solid Dosage Contract Manufacturing Market for Stick Packs, till 2035
- 16.2.4. Oral Solid Dosage Contract Manufacturing Market, till 2035: Distribution by Scale of Operation
- 16.2.4.1. Oral Solid Dosage Contract Manufacturing Market for Pre-Commercial Scale, till 2035
- 16.2.4.2. Oral Solid Dosage Contract Manufacturing Market for Commercial Scale, till 2035
- 16.2.5. Oral Solid Dosage Contract Manufacturing Market, till 2035: Distribution by Company Size
- 16.2.5.1. Oral Solid Dosage Contract Manufacturing Market for Small Companies, till 2035
- 16.2.5.2. Oral Solid Dosage Contract Manufacturing Market for Mid-sized Companies, till 2035
- 16.2.5.3. Oral Solid Dosage Contract Manufacturing Market for Large Companies, till 2035
- 16.2.5.4. Oral Solid Dosage Contract Manufacturing Market for Very Large Companies, till 2035
- 16.2.6. Oral Solid Dosage Contract Manufacturing Market, till 2035: Distribution by Therapeutic Area
- 16.2.6.1. Oral Solid Dosage Contract Manufacturing Market for Oncological Disorders, till 2035
- 16.2.6.2. Oral Solid Dosage Contract Manufacturing Market for Infectious Diseases, till 2035
- 16.2.6.3. Oral Solid Dosage Contract Manufacturing Market for Cardiovascular Disorders, till 2035
- 16.2.6.4. Oral Solid Dosage Contract Manufacturing Market for Metabolic Disorders, till 2035
- 16.2.6.5. Oral Solid Dosage Contract Manufacturing Market for Neurological Disorders, till 2035
- 16.2.6.6. Oral Solid Dosage Contract Manufacturing Market for Genetic Disorders, till 2035
- 16.2.6.7. Oral Solid Dosage Contract Manufacturing Market for Respiratory Disorders, till 2035
- 16.2.6.8. Oral Solid Dosage Contract Manufacturing Market for Immunological Disorders, till 2035
- 16.2.6.9. Oral Solid Dosage Contract Manufacturing Market for Other Disorders, till 2035
- 16.2.7. Oral Solid Dosage Contract Manufacturing Market, till 2035: Distribution by Geographical Regions
- 16.2.7.1. Oral Solid Dosage Contract Manufacturing Market in North America, till 2035
- 16.2.7.2. Oral Solid Dosage Contract Manufacturing Market in Europe, till 2035
- 16.2.7.3. Oral Solid Dosage Contract Manufacturing Market in Asia-Pacific, till 2035
- 16.2.7.4. Oral Solid Dosage Contract Manufacturing Market in Latin America, till 2035
- 16.2.7.5. Oral Solid Dosage Contract Manufacturing Market in Middle East and North Africa, till 2035



















