
|
시장보고서
상품코드
1737051
미생물 발효 CMO 시장 : 발현 시스템 유형별, 생물 제제 유형별, 기업 규모별, 사용자별, 주요 지역별Microbial Fermentation CMO Market Distribution by Type of Expression System, Type of Biologic, Company Size, End Users and Key Geographical Regions |
||||||
세계 미생물 발효 CMO 시장 규모는 2035년까지 예측 기간 동안 8.7%의 연평균 복합 성장률(CAGR)로, 36억 달러에서 2035년까지 91억 달러로 성장할 것으로 예측됩니다.
시장 세분화는 시장 규모와 기회 분석을 다음 매개 변수로 구분합니다.
발현 시스템 유형별
- 박테리아 발현 시스템
- 효모 발현 시스템
- 기타 발현 시스템
생물제제 유형별
- 재조합 단백질
- 효소
- 플라스미드 DNA
- 항체
- 기타 생물제제
기업 규모별
- 소규모
- 중규모
- 대기업 및 초대형
최종 사용자별
- 제약/바이오테크놀러지 기업
- 학술/연구기관
주요 지역별
- 북미
- 유럽
- 아시아태평양
- 기타 지역
미생물 발효 CMO 시장 : 성장과 동향
최근 미생물 발효 분야는 르네상스를 경험하고 있으며, 미생물 바이오 의약품 산업은 첨단 미생물 발현 시스템을 통한 생물 제제의 생산에 점점 더 초점을 맞추었습니다. 물 발효에 기초한 발현 시스템의 개선 및 혁신은 생물학적 제제의 부적절한 3차원 폴딩과 같은 불활성 또는 유해한 단백질로 이어질 수 있는 미생물 발효와 관련된 이전의 우려의 일부 미생물 발효 시스템은 고품질의 생물 제제를 대량으로 수탁 제조하는 것을 용이하게 했습니다. 생물 발효 기업이 제공하는 혁신적인 기술과 플랫폼의 진보로 인한 것입니다.
현재 다양한 생물 제제, 특히 사이토 카인, 성장 인자, 플라스미드 DNA, 핵산, 단일 도메인 항체, 비 글리코 실화 항체 단편 등의 저분자를 생산하기 위해 박테리아, 진균 및 효모를 이용한 전통적이고 고급 미생물 발현 스템/플랫폼이 광범위하게 채용되고 있으며, 그 결과 미생물 발효는 수탁 서비스 제공업체에게 유리한 시장 성장 기회를 가져올 가능성이 높고, 미생물 발효 CMO 시장은 향후 수년간 급성장할 것으로 예측됩니다.
미생물 발효 CMO 시장 : 주요 인사이트력
이 보고서는 미생물 발효 CMO 시장의 현재 상태를 파악하고 업계 내 잠재적 성장 기회를 확인합니다.
- 현재 120개 이상의 기업이 세계 미생물 발효 수탁 제조 서비스를 제공하고 있으며, 이들 기업의 대부분은 유럽에 본사를 두고 있습니다.
- 미생물 발효 CMO의 현재 시장 상황은 단편화되어 있으며 신규 진출기업과 기존 기업 모두 존재합니다.
- 생물 제제에 대한 수요가 높아짐에 따라 서비스 제공업체와 의약품 혁신자는 미생물 발현 시스템을 이용하여 고품질의 제품을 효율적으로 제조하기 위한 기술적 전문 지식을 향상시키고 있습니다.
- 이 영역에 대한 관심이 높아지고 있는 것은 제휴 활동 증가로부터도 분명합니다.
- 업계 각 사는 임상 스케일과 상업 스케일 모두 미생물 발효를 위해 기존의 능력과 능력을 확대하기 위한 광범위한 노력을 실시했습니다.
- 미생물 발효 CMO 시장은 2035년까지 연평균 복합 성장률(CAGR) 8.7%로 성장할 것으로 보입니다. 현재 시장 점유율의 대부분은 업스트림 세균 발현 시스템이 차지하고 있습니다.
- 미생물 발효 수탁 제조 시장은 주로 생물 제제 수요 증가별 견인될 것으로 예측되며, 2035년까지 유럽이 시장의 큰 점유율(40% 이상)을 차지할 것으로 예측됩니다.
미생물 발효 CMO 시장 : 주요 부문
발현 시스템 유형별로는 시장은 세균 발현 시스템, 효모 발현 시스템, 기타로 구분됩니다. 현재, 세균 발현계 부문은 배양에 있어서의 비용 대 효과, 유전자 조작의 용이함, 재조합 단백질의 신속한 생산에 의해 세계의 미생물 발효 CMO 시장에서 최대의 점유율을 차지하고 있습니다.
생물 제제 유형별로, 시장은 재조합 단백질, 효소, 플라스미드 DNA, 항체 등으로 구분됩니다. 현재 세계의 미생물 발효 CMO 시장에서 가장 높은 비율을 차지하고 있는 것은 재조합 단백질입니다.
기업 규모별로 시장은 중소, 중견, 대기업, 초대형으로 구분됩니다. 현재 미생물 발효 CMO 시장에서는 대기업과 초대형 수탁 제조업자가 최대 점유율을 차지하고 있습니다.
최종 사용자별로 세계 시장은 제약/바이오테크놀러지 기업과 학술/연구기관으로 구분됩니다.
주요 지역별로 볼 때 시장은 북미, 유럽, 아시아태평양 및 기타 지역으로 구분됩니다. 현재 유럽이 세계 미생물 발효 CMO 시장을 독점하고 있으며, 최대 수익 점유율을 차지하고 있습니다.
본 보고서에서는 세계의 미생물 발효 CMO 시장에 대해 조사했으며,, 시장 개요와 함께 발현 시스템 유형별, 생물 제제 유형별, 기업 규모별, 사용자별, 주요 지역별 동향, 시장 진출기업 프로파일 등의 정보를 제공합니다.
목차
제1장 서문
제2장 조사 방법
제3장 경제적 및 기타 프로젝트 특유의 고려 사항
제4장 주요 요약
제5장 소개
- 장의 개요
- 발현 시스템 유형
- 미생물 제조 공정의 단계
- 계약 제조 개요
- 미생물 제조 아웃소싱의 필요성
- 미생물 계약 제조 업계에서 일반적으로 아웃소싱되는 업무
- 제조 서비스의 아웃소싱에 따른 장단점
- CMO 파트너를 선택할 때 고려해야 할 중요한 점
제6장 시장 상황: 미생물 발효 수탁 제조업자
- 장의 개요
- 미생물 발효 수탁 제조 업체 : 시장 상황
제7장 기업 경쟁력 분석
제8장 기업 프로파일
- 장의 개요
- 주요 진출기업 프로파일
- AGC Biologics
- Aldevron
- BioVectra
- EirGenix
- EtinPro
- Eurogentec
- Northway Biotech
- Stelis Biosource
- 기타 기업 프로파일
- 53Biologics
- AbbVie Contract Manufacturing
- Ajinomoto Bio-Pharma Services
- Aumgene Biosciences
- BioCina
- Creative Biogene
- Eubiologics
- Eurofins
- FUJIFILM Diosynth Biotechnologies
- KBI Biopharma
- Lonza
- Menarini Biotech
- Mycenax Biotech
- WACKER
- WuXi Biologics
제9장 파트너십 및 협업
- 장의 개요
- 파트너십 모델
- 미생물 발효 CMO: 파트너십 및 협업
제10장 최근 확대
제11장 대기업 제약회사의 미생물 제조에 대한 대처
- 장의 개요
- 주요 제약 회사의 미생물 바이오 의약품 제조 이니셔티브 목록
- 주요 제약 회사의 경쟁 벤치마킹
제12장 매력도 경쟁 매트릭스
제13장 제조 또는 구입의 의사결정의 틀
제14장 사례 연구 I: 미생물 발효 기술 플랫폼
제15장 사례 연구 II: 소분자제와 대분자제/치료법의 비교
제16장 시장 영향 분석 : 촉진요인, 억제요인, 기회, 과제
제17장 세계의 미생물 발효 CMO 시장
- 장의 개요
- 주요 전제와 조사 방법
- 세계의 미생물 발효 CMO 시장, 과거 동향(2018년 이후) 및 예측(2035년까지)
- 주요 시장 세분화
제18장 미생물 발효 CMO 시장(사용되는 발현 시스템 유형별)
제19장 미생물 발효 CMO 시장(생물 제제 유형별)
제20장 미생물 발효 CMO 시장(기업 규모별)
제21장 미생물 발효 CMO 시장(최종사용자별)
제22장 미생물 발효 CMO 시장(주요 지역별)
제23장 결론
제24장 주요 인사이트
제25장 부록 1:표 형식 데이터
제26장 부록 2 : 기업·단체 일람
SHW 25.06.09MICROBIAL FERMENTATION CMO MARKET: OVERVIEW
As per Roots Analysis, the global microbial fermentation CMO market is estimated to grow from USD 3.6 billion in the current year to USD 9.1 billion by 2035, at a CAGR of 8.7% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Expression System
- Bacterial Expression Systems
- Yeast Expression Systems
- Other Expression Systems
Type of Biologic
- Recombinant Proteins
- Enzymes
- Plasmid DNA
- Antibodies
- Other Biologics
Company Size
- Small
- Mid-Sized
- Large and Very Large
End Users
- Pharma / Biotech Companies
- Academic / Research Institutes
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
MICROBIAL FERMENTATION CMO MARKET: GROWTH AND TRENDS
In recent years, the field of microbial fermentation has experienced a renaissance, with the microbial biopharmaceutical industry increasingly focusing on the production of biologics through advanced microbial expression systems. The modification and innovation in microbial fermentation-based expression systems for the production of various biologics have sidelined some of the earlier concerns associated with them like improper 3D folding of the biologics that can lead to inactive or even harmful proteins. As a result, microbial fermentation systems have facilitated the contract manufacturing of high-quality biologics in larger quantity. Further, the increasing adoption of microbial fermentation for the production of biologics has been driven by the advancements in innovative technologies and platforms offered by leading microbial fermentation companies. Over time, the microbial fermentation domain has significantly evolved and has led to the production of various biologics, primarily proteins.
Currently, a wide range of traditional and advanced microbial expression systems / platforms, utilizing bacteria, fungi, and yeast, are employed to produce diverse biologics, particularly smaller molecules such as cytokines, growth factors, plasmid DNA, nucleic acids, single-domain antibodies, and non-glycosylated antibody fragments. Consequently, microbial fermentation is likely to present lucrative market growth opportunities for contract service providers and the microbial fermentation CMO market is anticipated to grow faster in the coming years.
MICROBIAL FERMENTATION CMO MARKET: KEY INSIGHTS
The report delves into the current state of the microbial fermentation CMO market and identifies potential growth opportunities within the industry. Some key findings from the report include:
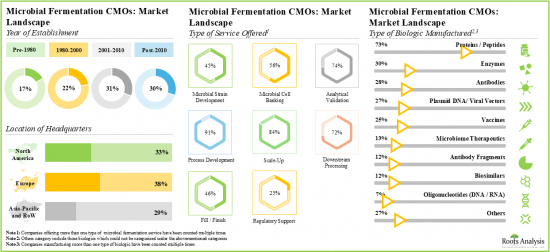
- Presently, more than 120 players are engaged in offering microbial fermentation contract manufacturing services across the globe; most of these players are headquartered in Europe.
- The current market landscape of microbial fermentation CMOs is fragmented, featuring the presence of both new entrants and established players; majority of these players offer process development services.
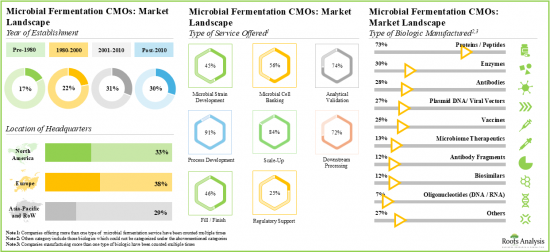
- Owing to the rise in demand for biologics, service providers and drug innovators are upgrading their technical expertise to efficiently manufacture high-quality products using microbial expression systems.
- The growing interest in this domain is evident from the rise in partnership activity; in fact, the maximum number of collaborations related to microbial fermentation were inked in the last two years.
- Industry players are making extensive efforts to expand their existing capacities and capabilities for both clinical and commercial scale microbial fermentation.
- The microbial fermentation CMO market is likely to grow at a CAGR of 8.7%, till 2035; presently, majority of the market share is occupied by upstream bacterial expression system.
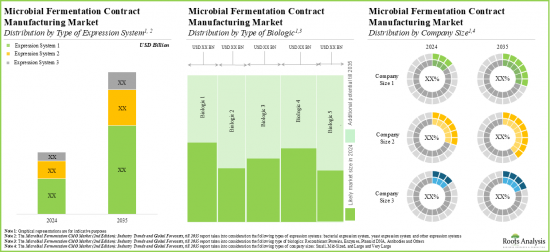
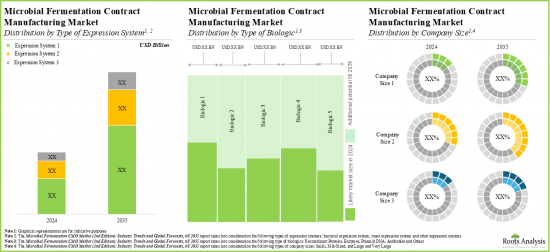
- The microbial fermentation contract manufacturing market is anticipated to be primarily driven by the rising demand for biologics; Europe is expected to capture a larger share (over 40%) of the market by 2035
MICROBIAL FERMENTATION CMO MARKET: KEY SEGMENTS
Bacterial Expression System Segment Occupies the Largest Share of the Microbial Fermentation CMO Market
Based on the type of expression system, the market is segmented into bacterial expression systems, yeast expression systems and other expression systems. At present, the bacterial expression systems segment holds the maximum share of the global microbial fermentation CMO market owing to its cost-effectiveness in culturing, ease of genetic manipulation, and fast production of recombinant proteins. Additionally, the yeast expression systems segment is likely to grow at a faster pace during the forecasted period.
By Type of Biologic, Plasmid DNA is the Fastest Growing Segment of the Global Microbial Fermentation CMO Market
Based on the type of biologic, the market is segmented into recombinant proteins, enzymes, plasmid DNA, antibodies and other biologics. Currently, the recombinant proteins segment captures the highest proportion of the global microbial fermentation CMO market. Further, the microbial fermentation CMO market for plasmid DNA is likely to grow at a relatively higher CAGR during the forecast period. This can be attributed to the increasing demand for the next- generation therapeutics.
Large and Very Large Contract Manufacturers Occupy the Largest Share of the Microbial Fermentation CMO Market by Company Size
Based on the company size, the market is segmented into small, mid-sized, large and very large companies. At present, large and very large contract manufacturers hold the maximum share of the microbial fermentation CMO market. Further, it is worth highlighting that the microbial fermentation CMO market for the mid-sized players segment is likely to grow at a relatively higher CAGR.
Currently, the Pharma / Biotech Companies Segment Holds the Largest Share of the Microbial Fermentation CMO Market
Based on the end users, the global market is segmented into pharma / biotech companies and academic / research institutes. Currently, the pharma / biotech companies segment holds the largest market share. In fact, this trend is likely to remain the same in the coming future.
Europe Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific and Rest of the World. Currently, Europe dominates the global microbial fermentation CMO market and accounts for the largest revenue share. Further, the market in Asia-Pacific is expected to grow at a higher CAGR in the coming years.
Example Players in the Microbial Fermentation CMO Market
- AbbVie
- BOC Sciences
- Eurofins
- FUJIFILM Diosynth Biotechnologies
- GSK
- Lonza
- Sandoz
MICROBIAL FERMENTATION CMO MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global microbial fermentation CMO market, focusing on key market segments, including [A] type of expression system, [B] type of biologic, [C] company size, [D] end users and [E] key geographical regions.
- Market Landscape: A comprehensive evaluation of the companies engaged in microbial fermentation CMO market, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] location of headquarters, [D] types of services offered, [E] affiliated services, [F] AI support, [G] analytical techniques used, [H] therapeutic areas and [I] regulatory certifications / accreditations.
- Company Competitiveness Analysis: A comprehensive competitive analysis of service providers in the microbial fermentation industry, examining factors, such as [A] developer strength and [B] product portfolio strength.
- Company Profiles: In-depth profiles of key service providers engaged in the microbial fermentation market, focusing on [A] overview of the company, [B] financial information (if available), [C] service portfolio, and [D] recent developments and an informed future outlook.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the microbial fermentation market, based on several parameters, such as [A] year of partnership, [B] type of biologic, [D] type of partner, [E] most active players (in terms of the number of partnerships signed) and [F] geographical distribution of partnership activity.
- Recent Expansions: In-depth analysis of the various expansion initiatives undertaken by contract biomanufacturers in this domain, based on several parameters, such as [A] year of expansion, [B] type of expansion, [C] microbial fermentation capacity, [D] type of biologic manufactured, [E] location of expanded facility, [F] type of expansion, [G] most active players and [H] geography.
- Big Pharma Initiatives in Microbial Manufacturing: An insightful analysis of big pharma players initiatives, based on several parameters, such as [A] number of initiatives, [B] year of initiative, and [C] types of initiatives.
- Attractiveness Competition Matrix: A detailed discussion on proprietary 2X2 representation to assess the current market scenario across emerging and established market segments.
- Make Versus Buy Decision Making Framework: An insightful analysis of the various factors that biopharmaceutical therapeutics / drug developers should consider while deciding whether to manufacture their respective products in-house or engage the services of CMOs.
- Case Study 1: A detailed discussion on the microbial fermentation technology / platforms that are available in the market. Additionally, a detailed discussion on emerging microbial fermentation technologies.
- Case Study 2: A detailed discussion on the key characteristics of large and small molecule drugs, along with information on the steps involved in their respective manufacturing processes.
- Market Impact Analysis: A thorough analysis of various factors, such as drivers, restraints, opportunities, and existing challenges that are likely to impact market growth.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Introduction
- 1.2. Market Share Insights
- 1.3. Key Market Insights
- 1.4. Report Coverage
- 1.5. Key Questions Answered
- 1.6. Chapter Outlines
2.RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.3. Project Methodology
- 2.4. Forecast Methodology
- 2.5. Robust Quality Control
- 2.6. Key Market Segmentations
- 2.7. Key Considerations
- 2.7.1. Demographics
- 2.7.2. Economic Factors
- 2.7.3. Government Regulations
- 2.7.4. Supply Chain
- 2.7.5. COVID Impact
- 2.7.6. Market Access
- 2.7.7. Healthcare Policies
- 2.7.8. Industry Consolidation
3. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Future Estimates
- 3.2.2. Currency Coverage and Foreign Exchange Rates
- 3.2.2.1. Major Currencies Affecting the Market
- 3.2.2.2. Factors Affecting Currency Fluctuations and Foreign Exchange Rates
- 3.2.2.3. Impact of Foreign Exchange Rate Volatility on the Market
- 3.2.2.4. Strategies for Mitigating Foreign Exchange Risk
- 3.2.3. Trade Policies
- 3.2.3.1. Impact of Trade Barriers on the Market
- 3.2.3.2. Strategies for Mitigating the Risks Associated with Trade Barriers
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. EXECUTIVE SUMMARY
- 4.1. Chapter Overview
5. INTRODUCTION
- 5.1. Chapter Overview
- 5.2. Types of Expression Systems
- 5.2.1. Microbial Expression Systems
- 5.2.1.1. Bacterial Expression Systems
- 5.2.1.2. Fungal Expression Systems
- 5.2.1.3. Yeast Expression Systems
- 5.2.1.4. Mammalian versus Microbial Expression Systems
- 5.2.1. Microbial Expression Systems
- 5.3. Stages of the Microbial Manufacturing Process
- 5.3.1. Cell Banking
- 5.3.2. Upstream Bioprocessing
- 5.3.3. Fermentation
- 5.3.4. Downstream Bioprocessing
- 5.4. Overview of Contract Manufacturing
- 5.5. Need for Outsourcing Microbial Manufacturing
- 5.6. Commonly Outsourced Operations in Microbial Contract Manufacturing Industry
- 5.7. Advantages and Disadvantages Associated with Outsourcing Manufacturing Services
- 5.8. Key Considerations while Selecting a CMO Partner
6. MARKET LANDSCAPE: MICROBIAL FERMENTATION CONTRACT MANUFACTURERS
- 6.1. Chapter Overview
- 6.2. Microbial Fermentation Contract Manufacturers: Overall Market Landscape
- 6.2.1. Analysis by Year of Establishment
- 6.2.2. Analysis by Company Size
- 6.2.3. Analysis by Location of Headquarters
- 6.2.4. Analysis by Type of Microbial Fermentation Service Offered
- 6.2.5. Analysis by Type of Microbial Expression System Used
- 6.2.6. Analysis by GMP Compliance
- 6.2.7. Analysis by Scale of Operation
- 6.2.8. Analysis by Type of Biologic Manufactured
- 6.2.9. Analysis by Type of Fermenter
- 6.2.10. Analysis by Location of Microbial Fermentation Facilities
7. COMPANY COMPETITIVENESS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Assumptions and Key Parameters
- 7.3. Methodology
- 7.4. Company Competitiveness Analysis: Microbial Fermentation Contract Manufacturers
- 7.4.1. Players based in North America (Peer Group I)
- 7.4.2. Players based in Europe (Peer Group II)
- 7.4.3. Players based in Asia-Pacific and Rest of the World (Peer Group III)
8. COMPANY PROFILES
- 8.1. Chapter Overview
- 8.2. Detailed Profiles of Leading Players
- 8.2.1. AGC Biologics
- 8.2.1.1. Company Overview
- 8.2.1.2. Microbial Fermentation Service Portfolio
- 8.2.1.3. Microbial Fermentation Facilities
- 8.2.1.4. Recent Developments and Future Outlook
- 8.2.2. Aldevron
- 8.2.2.1. Company Overview
- 8.2.2.2. Microbial Fermentation Service Portfolio
- 8.2.2.3. Microbial Fermentation Facilities
- 8.2.2.4. Recent Developments and Future Outlook
- 8.2.3. BioVectra
- 8.2.3.1. Company Overview
- 8.2.3.2. Microbial Fermentation Service Portfolio
- 8.2.3.3. Microbial Fermentation Facilities
- 8.2.3.4. Recent Developments and Future Outlook
- 8.2.4. EirGenix
- 8.2.4.1. Company Overview
- 8.2.4.2. Financial Information
- 8.2.4.3. Microbial Fermentation Service Portfolio
- 8.2.4.4. Microbial Fermentation Facilities
- 8.2.4.5. Recent Developments and Future Outlook
- 8.2.5. EtinPro
- 8.2.5.1. Company Overview
- 8.2.5.2. Microbial Fermentation Service Portfolio
- 8.2.5.3. Microbial Fermentation Facilities
- 8.2.5.4. Recent Developments and Future Outlook
- 8.2.6. Eurogentec
- 8.2.6.1. Company Overview
- 8.2.6.2. Microbial Fermentation Service Portfolio
- 8.2.6.3. Microbial Fermentation Facilities
- 8.2.6.4. Recent Developments and Future Outlook
- 8.2.7. Northway Biotech
- 8.2.7.1. Company Overview
- 8.2.7.2. Microbial Fermentation Service Portfolio
- 8.2.7.3. Microbial Fermentation Facilities
- 8.2.7.4. Recent Developments and Future Outlook
- 8.2.8. Stelis Biosource
- 8.2.8.1. Company Overview
- 8.2.8.2. Microbial Fermentation Service Portfolio
- 8.2.8.3. Microbial Fermentation Facilities
- 8.2.8.4. Recent Developments and Future Outlook
- 8.2.1. AGC Biologics
- 8.3. Short Profiles of Other Prominent Players
- 8.3.1. 53Biologics
- 8.3.1.1. Company Overview
- 8.3.1.2. Microbial Fermentation Service Portfolio
- 8.3.1.3. Microbial Fermentation Facilities
- 8.3.2. AbbVie Contract Manufacturing
- 8.3.2.1. Company Overview
- 8.3.2.2. Microbial Fermentation Service Portfolio
- 8.3.2.3. Microbial Fermentation Facilities
- 8.3.3. Ajinomoto Bio-Pharma Services
- 8.3.3.1. Company Overview
- 8.3.3.2. Microbial Fermentation Service Portfolio
- 8.3.3.3. Microbial Fermentation Facilities
- 8.3.4. Aumgene Biosciences
- 8.3.4.1. Company Overview
- 8.3.4.2. Microbial Fermentation Service Portfolio
- 8.3.4.3. Microbial Fermentation Facilities
- 8.3.5. BioCina
- 8.3.5.1. Company Overview
- 8.3.5.2. Microbial Fermentation Service Portfolio
- 8.3.5.3. Microbial Fermentation Facilities
- 8.3.6. Creative Biogene
- 8.3.6.1. Company Overview
- 8.3.6.2. Microbial Fermentation Service Portfolio
- 8.3.6.3. Microbial Fermentation Facilities
- 8.3.7. Eubiologics
- 8.3.7.1. Company Overview
- 8.3.7.2. Microbial Fermentation Service Portfolio
- 8.3.7.3. Microbial Fermentation Facilities
- 8.3.8. Eurofins
- 8.3.8.1. Company Overview
- 8.3.8.2. Microbial Fermentation Service Portfolio
- 8.3.8.3. Microbial Fermentation Facilities
- 8.3.9. FUJIFILM Diosynth Biotechnologies
- 8.3.9.1. Company Overview
- 8.3.9.2. Microbial Fermentation Service Portfolio
- 8.3.9.3. Microbial Fermentation Facilities
- 8.3.10. KBI Biopharma
- 8.3.10.1. Company Overview
- 8.3.10.2. Microbial Fermentation Service Portfolio
- 8.3.10.3. Microbial Fermentation Facilities
- 8.3.11. Lonza
- 8.3.11.1. Company Overview
- 8.3.11.2. Microbial Fermentation Service Portfolio
- 8.3.11.3. Microbial Fermentation Facilities
- 8.3.12. Menarini Biotech
- 8.3.12.1. Company Overview
- 8.3.12.2. Microbial Fermentation Service Portfolio
- 8.3.12.3. Microbial Fermentation Facilities
- 8.3.13. Mycenax Biotech
- 8.3.13.1. Company Overview
- 8.3.13.2. Microbial Fermentation Service Portfolio
- 8.3.13.3. Microbial Fermentation Facilities
- 8.3.14. WACKER
- 8.3.14.1. Company Overview
- 8.3.14.2. Microbial Fermentation Service Portfolio
- 8.3.14.3. Microbial Fermentation Facilities
- 8.3.15. WuXi Biologics
- 8.3.15.1. Company Overview
- 8.3.15.2. Microbial Fermentation Service Portfolio
- 8.3.15.3. Microbial Fermentation Facilities
- 8.3.1. 53Biologics
9. PARTNERSHIPS AND COLLABORATIONS
- 9.1. Chapter Overview
- 9.2. Partnership Models
- 9.3. Microbial Fermentation CMO: Partnerships and Collaborations
- 9.3.1. Analysis by Year of Partnerships
- 9.3.2. Analysis by Type of Partnership
- 9.3.3. Analysis by Year and Type of Partnership
- 9.3.4. Analysis by Type of Biologic Manufactured
- 9.3.5. Analysis by Type of Partner
- 9.3.6. Most Active Players: Distribution by Number of Partnerships
- 9.3.7. Analysis by Geography
- 9.3.7.1. Intracontinental and Intercontinental Agreements
- 9.3.7.2. International and Local Deals
10. RECENT EXPANSIONS
- 10.1. Chapter Overview
- 10.2. Microbial Fermentation CMO: Recent Expansions
- 10.2.1. Analysis by Year of Expansion
- 10.2.2. Analysis by Type of Expansion
- 10.2.3. Analysis by Year and Type of Expansion
- 10.2.4. Analysis by Microbial Fermentation Capacity
- 10.2.5. Analysis by Type of Biologic Manufactured
- 10.2.6. Analysis by Location of Expanded Facility
- 10.2.7. Analysis by Type of Expansion Location of Expanded Facility
- 10.2.8. Most Active Players: Distribution by Number of Recent Expansions
- 10.2.9. Analysis by Geography
- 10.2.9.1. Analysis by Continent
- 10.2.9.2. Analysis by Country
11. BIG PHARMA INITITATIVES IN MICROBIAL MANUFACTURING
- 11.1. Chapter Overview
- 11.2. List of Microbial Biopharmaceutical Manufacturing Initiatives of Big Pharma Players
- 11.2.1. Analysis by Number of Initiatives
- 11.2.2. Analysis by Year of Initiative Undertaken
- 11.2.3. Analysis by Company and Year of Initiative Undertaken
- 11.2.4. Analysis by Purpose of Initiative Undertaken
- 11.2.5. Analysis by Company and Purpose of Initiative Undertaken
- 11.2.6. Analysis by Type of Initiative
- 11.2.7. Analysis by Scale of Operation
- 11.2.8. Analysis by Type of Drug Molecule
- 11.2.9. Analysis by Type of Microbial Expression System Used
- 11.2.10. Geographical Analysis by Amount Invested
- 11.3. Competitive Benchmarking of Big Pharmaceutical Players
- 11.3.1. Concluding Remarks
12. ATTRACTIVENESS COMPETITION MATRIX
- 12.1. Chapter Overview
- 12.2. AC Matrix: An Overview
- 12.2.1. Strong Business Segment
- 12.2.2. Average Business Segment
- 12.2.3. Weak Business Segment
- 12.3. AC Matrix: Analytical Methodology
- 12.4. AC Matrix: Contract Manufacturing Scenario in North America
- 12.5. AC Matrix: Contract Manufacturing Scenario in Europe
- 12.6. AC Matrix: Contract Manufacturing Scenario in Asia Pacific and Middle East
13. MAKE VERSUS BUY DECISION MAKING FRAMEWORK
- 13.1. Chapter Overview
- 13.2. Assumptions and Parameter Definitions
- 13.2.1. Scenario 1
- 13.2.2. Scenario 2
- 13.2.3. Scenario 3
- 13.2.4. Scenario 4
- 13.3. Concluding Remarks
14. CASE STUDY I: MICROBIAL FERMENTATION TECHNOLOGY PLATFORMS
- 14.1. Chapter Overview
- 14.2. Technology Platforms Commonly Used for Microbial Fermentation
- 14.3. Upcoming Platforms-General Technologies
15. CASE STUDY II: COMPARISON OF SMALL MOLECULE AND LARGE MOLECULE DRUGS / THERAPIES
- 15.1. Chapter Overview
- 15.2. Small Molecule and Large Molecule Drugs / Therapies
- 15.2.1. Comparison of Key Characteristics
- 15.2.2. Comparison of Microbial Manufacturing Process
- 15.2.2.1. Microbial Contract Manufacturers Providing Services for Small Molecules
- 15.2.3. Comparison of Key Manufacturing-related Challenges
16. MARKET IMPACT ANALYSIS: DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES
- 16.1. Chapter Overview
- 16.2. Market Drivers
- 16.3. Market Restraints
- 16.4. Market Opportunities
- 16.5. Market Challenges
- 16.6. Conclusion
17. GLOBAL MICROBIAL FERMENTATION CMO MARKET
- 17.1. Chapter Overview
- 17.2. Key Assumptions and Methodology
- 17.3. Global Microbial Fermentation CMO Market, Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 17.3.1. Scenario Analysis
- 17.3.1.1. Conservative Scenario
- 17.3.1.2. Optimistic Scenario
- 17.3.1. Scenario Analysis
- 17.4. Key Market Segmentations
18. MICROBIAL FERMENTATION CMO MARKET, BY TYPE OF EXPRESSION SYSTEM USED
- 18.1. Chapter Overview
- 18.2. Key Assumptions and Methodology
- 18.3. Microbial Fermentation CMO Market: Distribution by Type of Expression System Used
- 18.3.1. Bacterial Expression Systems Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 18.3.2. Yeast Expression Systems Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 18.3.3. Other Expression Systems Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 18.4. Data Triangulation and Validation
19. MICROBIAL FERMENTATION CMO MARKET, BY TYPE OF BIOLOGIC
- 19.1. Chapter Overview
- 19.2. Key Assumptions and Methodology
- 19.3. Microbial Fermentation CMO Market: Distribution by Type of Biologic
- 19.3.1. Recombinant Proteins Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 19.3.2. Enzymes Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 19.3.3. Plasmid DNA Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 19.3.4. Antibodies Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 19.3.5. Other Biologics Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 19.4. Data Triangulation and Validation
20. MICROBIAL FERMENTATION CMO MARKET, BY COMPANY SIZE
- 20.1. Chapter Overview
- 20.2. Key Assumptions and Methodology
- 20.3. Microbial Fermentation CMO Market: Distribution by Company Size
- 20.3.1. Small Companies: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 20.3.2. Mid-Sized Companies: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 20.3.3. Large and Very Large Companies: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 20.4. Data Triangulation and Validation
21. MICROBIAL FERMENTATION CMO MARKET, BY END-USERS
- 21.1. Chapter Overview
- 21.2. Key Assumptions and Methodology
- 21.3. Microbial Fermentation CMO Market: Distribution by End-User
- 21.3.1. Pharma / Biotech Companies Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 21.3.2. Academic / Research Institutes Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 21.4. Data Triangulation and Validation
22. MICROBIAL FERMENTATION CMO MARKET, BY KEY GEOGRAPHICAL REGIONS
- 22.1. Chapter Overview
- 22.2. Key Assumptions and Methodology
- 22.3. Microbial Fermentation CMO Market: Distribution by Key Geographical Regions
- 22.3.1. North America Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.1. Bacterial Expression Systems Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.2. Yeast Expression Systems Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.3. Other Expression Systems Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.4. Recombinant Proteins Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.5. Enzymes Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.6. Plasmid DNA Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.7. Antibodies Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.8. Other Biologics Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.9. Small Companies Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.10. Mid-Sized Companies Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.11. Large and Very Large Companies Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.12. Pharma / Biotech Companies Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1.13. Academic / Research Institutes Market in North America: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2. Europe Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.1. Bacterial Expression Systems Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.2. Yeast Expression Systems Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.3. Other Expression Systems Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.4. Recombinant Proteins Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.5. Enzymes Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.6. Plasmid DNA Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.7. Antibodies Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.8. Other Biologics Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.9. Small Companies Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.10. Mid-Sized Companies Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.11. Large and Very Large Companies Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.12. Pharma/ Biotech Companies Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.2.13. Academic/ Research Institutes Market in Europe: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3. Asia-Pacific Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.1. Bacterial Expression Systems Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.2. Yeast Expression Systems Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.3. Other Expression Systems Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.4. Recombinant Proteins Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.5. Enzymes Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.6. Plasmid DNA Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.7. Antibodies Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.8. Other Biologics Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.9. Small Companies Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.10. Mid-Sized Companies Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.11. Large and Very Large Companies Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.12. Pharma/ Biotech Companies Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.3.13. Academic/ Research Institutes Market in Asia-Pacific: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4. Rest of the World Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.1. Bacterial Expression Systems Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.2. Yeast Expression Systems Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.3. Other Expression Systems Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.4. Recombinant Proteins Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.5. Enzymes Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.6. Plasmid DNA Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.7. Antibodies Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.8. Other Biologics Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.9. Small Companies Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.10. Mid-Sized Companies Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.11. Large and Very Large Companies Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.12. Pharma/ Biotech Companies Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.4.13. Academic/ Research Institutes Market in Rest of the World: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.3.1. North America Market: Historical Trends (Since 2018) and Forecasted Estimates (Till 2035)
- 22.4. Data Triangulation and Validation
23. CONCLUDING REMARKS
- 23.1. Chapter Overview
24. EXECUTIVE INSIGHTS
- 24.1. Chapter Overview
- 24.2. Meteoric Biopharmaceuticals
- 24.2.1. Company Snapshot
- 24.2.2. Interview Transcript
- 24.3. List Biological Laboratories
- 24.3.1. Company Snapshot
- 24.3.2. Interview Transcript
- 24.4. OLON
- 24.4.1. Company Snapshot
- 24.4.2. Interview Transcript
- 24.5. Luina Bio
- 24.5.1. Company Snapshot
- 24.5.2. Interview Transcript
- 24.6. WACKER Biotech
- 24.6.1. Company Snapshot
- 24.6.2. Interview Transcript



















