
|
시장보고서
상품코드
1737053
RNAi 치료제 및 기술 시장 : 표적 치료 영역별, 투여 경로별, RNAi 분자 유형별, 지역별, 주요 기업별RNAi Therapeutics and Technology Market by Target Therapeutic Areas, Route of Administration, Type of RNAi Molecule, Geographical Regions and Leading Industry Players |
||||||
Roots Analysis에 따르면 세계 RNAi 치료제 및 기술 시장 규모는 2035년까지 예측 기간 동안 17%의 연평균 복합 성장률(CAGR)로, 12억 달러에서 2035년까지 92억 달러로 성장할 것으로 예측됩니다.
시장 세분화는 시장 규모와 기회 분석을 다음 매개 변수로 구분합니다.
대상 치료 영역별
- 유전자 질환
- 대사질환
- 종양학적 질환
- 유전자 질환
- 혈액 질환
- 안과 질환
- 기타
투여 경로별
- 피하
- 정맥내 투여
- 안과
- 피내
RNAi 분자 유형별
- siRNA
- shRNA
지역별
- 북미
- 유럽
- 아시아태평양 및 기타 지역
판매 예측
- 온 파트로(R)
- 지브러리(TM)
- 렉비오(R)
- 옥수르모(TM)
- 피투실란
- 브트리실란
- SYL-1001
- 비질-EWS
- 네도실란
RNAi 치료제 및 기술 시장 : 성장과 동향
RNAi 치료제는 유전자의 RNA 간섭(RNAi) 특성을 이용하여 세포 내에서 자연적으로 발현계를 제어하는 혁신적인 약제의 신클래스를 가리킵니다. RNAi의 유전자 특이성이 치료법의 개발에 사용되는 주된 이유입니다.
흥미롭게도, 2018년, 파티실란은 만성 질환으로 고통받는 환자를 치료하기 위해 FDA로부터 승인을 받은 최초의 RNAi 기반 치료제가 되었습니다. 그 이후로 기보시란(2019년), 루마실란(2020년), 잉크리실란(2021년), 부트리실란(2022년), 네드실란(2023년)이 신규 RNAi 기반 치료로 FDA로부터 승인을 받았습니다. 200개가 넘는 RNAi 치료제가 현재 광범위한 적응증 치료를 위해 다양한 임상시험에서 평가되고 있습니다. 또한 안티센스 접근법에 따른 효과성 향상과 이펙터 분자 농도의 저감을 목표로 RNA 간섭 기술 개발을 위한 연구 노력을 높이고 있는 기업도 있습니다.
RNAI 치료제 및 기술 시장 : 주요 인사이트력
이 보고서는 RNAi 치료제 및 기술 시장의 현재 상태를 파악하고 업계 내 잠재적 성장 기회를 확인합니다.
- 현재 200개가 넘는 RNAi 의약품 후보가 무수한 질환 적응증 치료제로서 다양한 개발 단계에서 연구되고 있으며, 이미 6개의 의약품이 승인을 받았습니다.
- 약제 후보의 80% 가까이가 siRNA 분자를 이용하여 개발되고 있으며, RNAi 약제의 40% 이상은 피하 전달용으로 설계되어 있습니다.
- RNAi 치료제의 개발에는 신흥기업 몇사를 포함한 45개 이상이 종사하고 있으며, 연구개발의 주요 기업은 미국에 있습니다.
- 이 시장은 오랜 기간 자리를 잡은 기업들의 존재로 특징지어지며, 상대적으로 경험이 적은 기업들 또한 초기 단계 후보 물질에 대한 강력한 파이프라인을 보유하고 있습니다.
- 급속히 발전하고 있는 이 영역에 대한 관심이 높아지고 있는 것은 최근 몇년에 체결된 제휴별 증명할 수 있습니다.
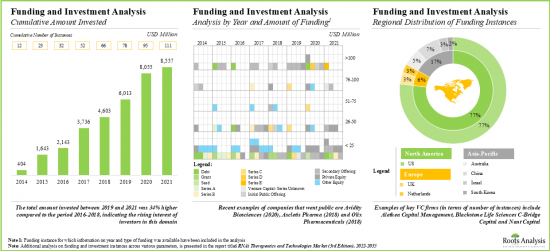
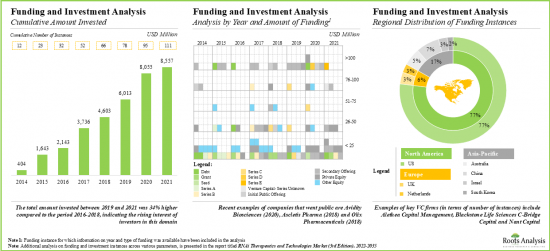
- 벤처캐피탈과 전략적 투자자는 이 유리한 기회를 이용하기 위해 현재 진행 중인 대처를 적극 지원하고 있으며 지난 몇 년간 110개 이상의 사례에 대해 85억 달러 이상이 투자되고 있습니다.
- 주목해야할 것은 등록된 임상시험이라는 점에서 현저한 임상활동입니다.
- 현재, RNAi 치료제의 임상 개발에 종사하고 있는 유명 대학의 많은 과학자가, 주요한 오피니언 리더로서 두각을 나타내고 있습니다.
- 현재, RNAi 치료제의 발견, 개발, 딜리버리를 위해, 55개 이상의 혁신적인 드래그 개발 및 딜리버리 기술 플랫폼이 업계 이해관계자별 활용되고 있습니다.
- 최근 몇 년간 발표된 과학문헌의 수가 순조롭게 성장하고 있는 것은 연구자들이 신규기술과 치료적 개입을 특정하고 개발하기 위해 집중하고 임하고 있음을 보여줍니다.
- RNAi 치료제과 관련 딜리버리 기술에 한해서 말하면, 과거 6년간에 2,100건 이상의 특허가 학계와 업계의 이해 관계자별 부여 및 출원되고 있습니다.
- 2035년에는 siRNA 의약품 수요의 60% 가까이가 다양한 유전성 질환을 앓고 있는 환자의 치료를 위해 북미에서 발생한다고 생각됩니다.
- 독자적인 제품을 효율적으로 수익화하기 위해, 의약품 개발 기업은 제품 출시 사이클의 다양한 단계에 적용할 수 있는 다양한 상업화 전략을 적극적으로 모색하고 있습니다.
- RNAi 치료제와 관련된 시장 기회는 향후 10년간 연률 17%의 성장이 예상됩니다.
- 전체적인 기회는 다양한 투여 경로, 시장 참가 기업, 나라 및 지역에 걸쳐서 잘 분산하고 있다고 생각됩니다.
RNAI 치료제 및 기술 시장 : 주요 부문
표적 치료 영역별로 시장은 종양성 질환, 유전성 질환, 대사성 질환, 혈액 질환, 안과 질환 등으로 구분됩니다.
투여 경로별로, 시장은 피하, 근육내, 안과, 피내 투여 경로로 구분됩니다. 현재, RNAi 치료제 및 기술 시장은 피하 투여 루트 부문이 세계의 RNAi 치료제 및 기술 시장에서 가장 높은 비율을 차지하고 있습니다.
RNAi 분자 유형별로 시장은 siRNA와 shRNA로 구분됩니다. 현재 RNAi 분자 유형별로 siRNA 부문이 세계 RNAi 치료제 및 기술 시장에서 가장 큰 점유율을 차지하고 있습니다. 특히 shRNA 부문은 비교적 높은 CAGR로 성장할 가능성이 높습니다.
지역별로 볼 때 시장은 북미, 유럽, 아시아태평양 및 기타 지역으로 구분됩니다.
RNAi 치료제 및 기술 시장에서 진출기업의 예
- AgelessRx
- ANOVA Institute for Regenerative Medicine
- Betterhumans
- BioAge Labs
- BioXcellerator
- Cambrian Biopharma
- Gero.ai
- Mayo Clinic
- Rejuvenate Bio
- UT Health San Antonio
본 보고서는 세계의 RNAi 치료제 및 기술 시장에 대해 조사했으며, 시장 개요와 함께 표적 치료 영역별, 투여 경로별, RNAi 분자 유형별, 지역별 동향, 시장 진출기업프로파일 등의 정보를 제공합니다.
목차
제1장 서문
제2장 주요 요약
제3장 소개
- 장의 개요
- 주요 이정표와 과거 동향
- RNAi의 메커니즘
- RNAi 분자의 유형
- RNAi의 응용
- RNAi의 장단점
- 장래의 전망
제4장 시장 개요
- 장의 개요
- RNAi 치료제 : 상시 완료 및 개발 파이프라인
제5장 경쟁 구도
- 장의 개요
- RNAi 치료제 : 개발자의 상황
- RNAi 치료제 : 지역정세
제6장 기업 경쟁력 분석
- 장의 개요
- 전제/주요 파라미터
- 조사 방법
- 기업 경쟁 분석 : RNAi 치료제 개발 기업
- 북미에 거점을 두는 개발자
- 유럽에 거점을 두는 개발자
- 아시아태평양에 거점을 두는 개발자
제7장 승인된 후기 단계의 RNAi 치료제
- 장의 개요
- 온 파트로(R)
- 기브라리(R)
- 렉비오(R)
- 옥수르모
- 피투실란
- 브트리실란
- SYL 1001
- 비질-EWS
- SR-061
- 네도실란
제8장 기술 플랫폼 및 배달 시스템
제9장 기술 경쟁력 분석
제10장 주요 치료 적응증
- 장의 개요
- 종양성 질환
- 감염증
- 대사 장애
- 안과 질환
- 유전성 질환
제11장 임상시험 분석
- 장의 개요
- 범위와 조사 방법
- RNAi 치료제: 임상시험 분석
- 주요 치료 후보
- 주요 임상시험
제12장 KOL(Key Opinion Leader)
제13장 특허 분석
제14장 출판물 분석
제15장 파트너십 및 협업
제16장 자금 조달과 투자 분석
제17장 주요 상업화 전략
제18장 진단에서의 RNAi
제19장 RNAi 치료 서비스 제공업체
제20장 수요 분석
제21장 시장 규모의 평가와 기회 분석
제22장 SWOT 분석
제23장 인터뷰 기록
제24장 결론
제25장 부록 1:표 형식 데이터
제26장 부록 2 : 기업·단체 일람
SHW 25.06.09RNAi THERAPEUTICS AND TECHNOLOGY MARKET: OVERVIEW
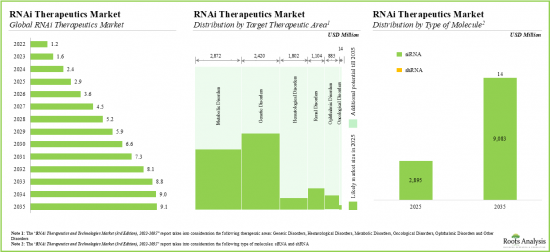
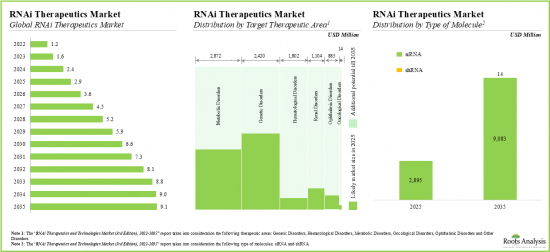
As per Roots Analysis, the global RNAi therapeutics and technology market is estimated to grow from USD 1.2 billion in the current year to USD 9.2 billion by 2035, at a CAGR of 17% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Target Therapeutic Areas
- Genetic Disorders
- Metabolic Disorders
- Oncological Disorders
- Genetic Disorders
- Hematological Disorders
- Ophthalmic Disorders
- Other Disorders
Route of Administration
- Subcutaneous
- Intravenous
- Ophthalmic
- Intradermal
Type of RNAi Molecule
- siRNA
- shRNA
Geographical Regions
- North America
- Europe
- Asia-Pacific and Rest of the World
Sales Forecast
- Onpattro(R)
- Givlaari(TM)
- Leqvio(R)
- Oxlumo(TM)
- Fitusiran
- Vutrisiran
- SYL-1001
- Vigil-EWS
- Nedosiran
RNAi THERAPEUTICS AND TECHNOLOGY MARKET: GROWTH AND TRENDS
RNAi therapeutics refer to the novel class of innovative drugs that harness the RNA interference (RNAi) property of genes for the regulation of the expression system naturally inside the cells. Fundamentally, RNAi is a natural process of post-transcriptional gene silencing, involving short strands of nucleic acids. Cells use this process to silence and / or inhibit gene expression, via targeted degradation of specific (unwanted) mRNA molecules. From an application perspective, the gene specificity of RNAi is the primary reason for it being used in the development of therapies. In theory, RNAi-based therapeutics are capable of treating a myriad of disease indications, such as age-related macular degeneration (AMD), hepatitis C and various forms of cancer, which are actually hard to treat, using conventional pharmacological options.
It is interesting to note that in, 2018, Patisiran became the first RNAi-based therapy to receive approval from the FDA to treat patients suffering from chronic diseases. Since then, six novel RNAi-based therapies, namely, Givosiran (2019), Lumasiran (2020), Inclisiran (2021), Vutrisiran (2022) and Nedosiran (2023), have received approval from the FDA. Notably, more than 200 RNAi therapeutics are currently being evaluated across different clinical trials for the treatment of a broad array of disease indications. Additionally, several industrial players are increasing their research efforts for the development of RNA interference technology, aiming to enhance efficacy and reduce effector molecule concentrations based on the anti-sense approach. Driven by ongoing research, it is estimated that the market will grow significantly during the forecast period.
RNAI THERAPEUTICS AND TECHNOLOGY MARKET: KEY INSIGHTS
The report delves into the current state of the RNAi therapeutics and technology market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Presently, more than 200 RNAi drug candidates are being investigated across various stages of development for the treatment of a myriad of disease indications; six such drug products have already received approval.
- Nearly 80% of the drug candidates are being developed using siRNA molecules; over 40% of the RNAi drugs are designed for delivery via the subcutaneous route.
- More than 45 companies, including several startups, are engaged in the development of RNAi therapeutics; the R&D efforts are being led by players located in the US.
- The market is characterized by the presence of well-established players; the relatively less experienced players also possess a robust pipeline of early-stage candidates.
- The rising interest in this rapidly evolving domain can be validated by the partnerships inked in recent years; the majority of the deals were signed for research and development purposes.
- In order to tap the lucrative opportunity, venture capital firms / strategic investors have actively supported the ongoing initiatives; over USD 8.5 billion has been invested across 110+ instances in the past few years.
- Notably, significant clinical activity, in terms of registered clinical trials, was observed; over 60% of the clinical studies are being conducted in parallel comparison of two groups of treatments.
- A number of scientists from renowned universities, presently involved in several clinical development efforts of RNAi therapeutics, have emerged as key opinion leaders.
- More than 55 innovative drug development and delivery technology platforms are presently being leveraged by industry stakeholders for the discovery, development and delivery of RNAi therapeutics.
- The healthy growth in published scientific literature over the last few years signifies the focused efforts being led by researchers to identify and develop novel technologies and therapeutic interventions.
- Over 2,100 patents have been granted / filed by academia and industry stakeholders in the last six years, exclusively for RNAi therapeutics and associated delivery technologies.
- In 2035, close to 60% of the demand for siRNA drugs is likely to be generated in North America for the treatment of patients suffering from various genetic disorders.
- In order to efficiently monetize their proprietary offerings, drug developers are actively exploring diverse commercialization strategies applicable across different stages of a product's launch cycle.
- The market opportunity associated with RNAi therapeutics is anticipated to witness an annualized growth rate of 17%, over the coming decade.
- The overall opportunity is likely to be well distributed across different routes of administration, market players and countries / regions.
RNAI THERAPEUTICS AND TECHNOLOGY MARKET: KEY SEGMENTS
Genetic Disorders Occupy the Largest Share of the Global RNAi Therapeutics and Technology Market
Based on the target therapeutic area, the market is segmented into oncological disorders, genetic disorders, metabolic disorders, hematological disorders, ophthalmic disorders and other disorders. At present, the genetic disorders segment holds the maximum share of the global RNAi therapeutics and technology market. This trend is likely to change in the forthcoming years.
By Route of Administration, RNAi Therapeutics and Technology focused on Intradermal Route of Administration is the Fastest Growing Segment of the Global RNAi Therapeutics and Technology Market During the Forecast Period
Based on the route of administration, the market is segmented into subcutaneous, intramuscular, ophthalmic and intradermal routes. Currently, the RNAi therapeutics and technology market is dominated by subcutaneous route of administration segment, capturing the highest proportion of the global RNAi therapeutics and technology market. It is worth highlighting that the global RNAi therapeutics and technology market for intradermal route of administration segment is likely to grow at a relatively higher CAGR.
siRNA Segment Occupies the Largest Share of the RNAi Therapeutics and Technology Market by Type of RNAi Molecule
Based on the type of RNAi molecule, the market is segmented into siRNA and shRNA. At present, siRNA segment by type of RNAi molecule holds the maximum share of the global RNAi therapeutics and technology market. Notably, shRNA segment is likely to grow at a relatively higher CAGR.
North America Accounts for the Largest Share of the Market
Based on the geographical regions, the market is segmented into North America, Europe, and Asia-Pacific and Rest of the World. The majority share is expected to be captured by players based in North America and Europe.
Example Players in the RNAi Therapeutics and Technology Market
- AgelessRx
- ANOVA Institute for Regenerative Medicine
- Betterhumans
- BioAge Labs
- BioXcellerator
- Cambrian Biopharma
- Gero.ai
- Mayo Clinic
- Rejuvenate Bio
- UT Health San Antonio
RNAI THERAPEUTICS AND TECHNOLOGY MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global RNAi therapeutics and technology market, focusing on key market segments, including [A] key therapeutic areas, [B] route of administration, [C] type of RNAi molecule, [D] geographical regions and [E] leading players.
- RNAi Therapeutics Market Landscape: A comprehensive evaluation of the RNAi pipeline candidates, based on several relevant parameters, such as [A] phase of development (marketed, clinical, and preclinical / discovery stage) of pipeline candidates, [B] target disease indication(s), [C] key therapeutic areas (oncological disorders, infectious diseases, genetic disorders, ophthalmic diseases, respiratory disorders, hepatic disorders, metabolic disorders, cardiovascular disorders, dermatological disorders and other disorders), [D] type of RNAi molecule (siRNA, miRNA, shRNA, shRNA and DNA), [E] target gene, [F] route of administration and [G] special drug designations (if any).
- RNAi Therapeutics Developers Market Landscape: A comprehensive evaluation of the companies engaged in this domain, based on several relevant parameters, such as [A] year of establishment, [B] company size and [C] location of headquarters.
- Company Competitiveness Analysis: A comprehensive competitive analysis of RNAi therapeutic developers, examining factors, such as [A] developer strength and [B] product portfolio strength.
- Drug Profiles: In-depth profiles of key RNAi therapeutic drugs, focusing on [A] current development status of the drug, [B] developer details, [C] mechanism of action, [D] route of administration, [E] affiliated technology / platform (if available), [F] dosage and [G] and recent clinical trial results.
- RNAi Therapeutics Technology Platforms Market Landscape: A comprehensive evaluation of the RNAi therapeutics technology platforms and their developers, based on several relevant parameters, such as [A] purpose of technology, [B] type of molecule (s) delivered and [C] type of cell (s) / tissue (s) targeted, [D] year of their establishment, [E] company size and [F] location of headquarters. Additionally, a brief overview of the profiles of key RNAi-based drug discovery / development technology platforms and drug delivery technologies (shortlisted on the basis of competitiveness score).
- Technology Competitiveness Analysis: A comprehensive competitive analysis of RNAi technologies, examining factors, such as [A] supplier power and [B] key technology specifications.
- Target Indications: A comprehensive analysis of target indications (segregated by various therapeutic areas) that are currently being focused on the companies engaged in the development of RNAi therapeutics.
- Clinical Trial Analysis: An insightful analysis of clinical studies, based on several parameters, such as [A] trial registration year, [B] current status, [C] phase of development, [D] type of RNAi molecule, [E] enrolled patient population and [F] regional distribution of trials.
- Key Opinion Leaders (KOLs]: An in-depth 2X2 analysis that emphasizes the key opinion leaders in this domain, shortlisted based on their contributions (in terms of involvement in various clinical studies) in this field.
- Patent Analysis: An in-depth analysis of patents filed / granted till date in the RNAi therapeutics domain, based on various relevant parameters, such as [A] type of patent, [B] publication year, [C] regional applicability, [D] CPC symbols, [E] emerging focus areas, [F] leading industry / non-industry players and [G] patent valuation.
- Publication Analysis: An insightful analysis of 3,000 peer-reviewed scientific articles in the RNAi therapeutics domain, based on various relevant parameters, such as [A] year of publication, [B] type of publication, [C] popular keywords, [D] top journals, [E] top publishers, [F] key copyright holders and [G] key funding institutes.
- Partnerships and Collaborations: An in-depth analysis of the deals inked by stakeholders in the RNAi therapeutics and technology market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] target disease indication, [D] therapeutic area, [E] type of RNAi molecule, [F] financial details (wherever applicable), [G] focus area of collaboration and [E] most active players (in terms of number of partnerships).
- Funding and Investment Analysis: An in-depth analysis of the fundings raised by RNAi therapeutics and technology companies, based on relevant parameters, such as [A] year of funding, [B] type of funding, [C] amount invested, [D] type of RNAi molecule, [E] most active players and [F] key investors.
- Case Study 1: A detailed discussion on the various commercialization strategies that can be adopted by the companies engaged in this domain, based on [A] different stages of therapy development, including prior to drug launch, at / during drug launch and post-marketing of the drug.
- Case Study 2: An in-depth discussion on the use of miRNA as a potential biomarker, along with a list of diagnostic kits that are either available in the market, or likely to be approved in the foreseen future.
- RNAi Therapeutics Service Providers Market Landscape: A comprehensive analysis of the companies that are actively supporting the development of RNAi therapeutics market, including contract manufacturers, contract researcher organizations and other service providers based on various parameters, such as [A] type of service provider, [B] location of their headquarters and [C] type of RNAi molecule developed.
- Demand Analysis: A detailed analysis of the annual clinical and commercial demand for RNAi therapeutics, based on the target patient population in ongoing and planned clinical trials of RNAi therapeutics, sponsored by both industry and non-industry players.
- SWOT Analysis: An analysis of industry affiliated trends, opportunities and challenges, which are likely to impact the evolution of RNAi therapeutics market; it includes a Harvey ball analysis, assessing the relative impact of each SWOT parameter on industry dynamics.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Market Segmentations
- 1.4. Key Questions Answered
- 1.5. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Key Milestones and Historical Trends
- 3.2.1. Discovery of RNAi
- 3.2.2. RNAi Therapy Development Efforts
- 3.3. Mechanism of RNAi
- 3.3.1. Components of RNAi
- 3.3.2. Cellular Mechanism
- 3.4. Types of RNAi Molecules
- 3.4.1. siRNA
- 3.4.2. miRNA
- 3.4.3. shRNA
- 3.5. Applications of RNAi
- 3.5.1. Functional Genomics
- 3.5.2. Therapeutics
- 3.5.3. Biotechnology
- 3.5.4. Genome-scale Screening
- 3.6. Advantages and Disadvantages of RNAi
- 3.6.1. Advantages of RNAi
- 3.6.2. Disadvantages of RNAi
- 3.6.3. Case Study: Concerns Discussed During Regulatory Submissions in Clinic
- 3.7. Future Perspectives
4. MARKET OVERVIEW
- 4.1. Chapter Overview
- 4.2. RNAi Therapeutics: Marketed and Development Pipeline
- 4.2.1. Analysis by Type of RNAi Molecule
- 4.2.2. Analysis by Phase of Development
- 4.2.3. Analysis by Type of Molecule and Phase of Development
- 4.2.4. Analysis by Target Gene
- 4.2.5. Analysis by Therapeutic Area
- 4.2.6. Analysis by Route of Administration
- 4.2.7. RNAi Therapeutics: Special Designations
- 4.2.8. Key Players
5. COMPETITIVE LANDSCAPE
- 5.1. Chapter Overview
- 5.2. RNAi Therapeutics: Developer Landscape
- 5.2.1. Analysis by Year of Establishment
- 5.2.2. Analysis by Company Size
- 5.2.3. Analysis by Location of Headquarters
- 5.2.4. Analysis by Location of Headquarters (Country-wise)
- 5.2.5. Key Players: Analysis by Number of Drug Candidates
- 5.3. RNAi Therapeutics: Regional Landscape
6. COMPANY COMPETITIVENESS ANALYSIS
- 6.1. Chapter Overview
- 6.2. Assumptions / Key Parameters
- 6.3. Methodology
- 6.4. Company Competitive Analysis: RNAi Therapeutics Developers
- 6.5. Developers based in North America
- 6.6. Developers based in Europe
- 6.7. Developers based in Asia-Pacific
7. APPROVED AND LATE-STAGE RNAi THERAPEUTICS
- 7.1. Chapter Overview
- 7.2. Onpattro(R)
- 7.2.1. Drug Overview
- 7.2.2. Technology Overview
- 7.2.3. Current Development Status
- 7.2.4. Recent Clinical Trial Results
- 7.2.5. Recent Partnerships
- 7.3. Givlaari(R)
- 7.3.1. Drug Overview
- 7.3.2. Technology Overview
- 7.3.3. Current Development Status
- 7.3.4. Recent Clinical Trial Results
- 7.3.5. Recent Partnerships
- 7.4. Leqvio(R)
- 7.4.1. Drug Overview
- 7.4.2. Technology Overview
- 7.4.3. Current Development Status
- 7.4.4. Recent Clinical Trial Results
- 7.4.5. Recent Partnerships
- 7.5. Oxlumo(TM)
- 7.5.1. Drug Overview
- 7.5.2. Technology Overview
- 7.5.3. Current Development Status
- 7.5.4. Recent Clinical Trial Results
- 7.5.5. Recent Partnerships
- 7.6. Fitusiran
- 7.6.1. Drug Overview
- 7.6.2. Technology Overview
- 7.6.3. Current Development Status
- 7.6.4. Recent Clinical Trial Results
- 7.7. Vutrisiran
- 7.7.1. Drug Overview
- 7.7.2. Technology Overview
- 7.7.3. Current Development Status
- 7.7.4. Recent Clinical Trial Results
- 7.8. SYL 1001
- 7.8.1. Drug Overview
- 7.8.2. Technology Overview
- 7.8.3. Current Development Status
- 7.8.4. Recent Clinical Trial Results
- 7.9. Vigil-EWS
- 7.9.1. Drug Overview
- 7.9.2. Technology Overview
- 7.9.3. Current Development Status
- 7.9.4. Recent Clinical Trial Results
- 7.10. SR-061
- 7.10.1. Drug Overview
- 7.10.2. Technology Overview
- 7.10.3. Current Development Status
- 7.11. Nedosiran
- 7.11.1. Drug Overview
- 7.11.2. Technology Overview
- 7.11.3. Current Development Status
- 7.11.4. Recent Clinical Trial Results
8. TECHNOLOGY PLATFORMS AND DELIVERY SYSTEMS
- 8.1. Chapter Overview
- 8.2. Components of RNAi Delivery Systems
- 8.2.1. RNAi Triggers
- 8.2.1.1. Asymmetric siRNA (cp-siRNA)
- 8.2.1.2. DNA Directed RNAi (ddRNAi)
- 8.2.1.3. Dicer Substrate siRNA (DsiRNA)
- 8.2.1.4. Naked siRNA
- 8.2.1.5. Self-Deliverable RNA (sd-RNA)
- 8.2.1.6. Self-Deliverable rxRNA (sd-rxRNA)
- 8.2.1.7. Unlocked Nucleobase Analog (UNA) Containing siRNA (UsiRNA)
- 8.2.2. Technology Platforms and Delivery Systems
- 8.2.2.1. Analysis by Purpose of Technology
- 8.2.2.2. Analysis by Type of Molecule (s) Delivered
- 8.2.2.3. Analysis by Type of Cell (s) / Tissue (s) Targeted
- 8.2.3. Technology Platforms: List of Developers
- 8.2.3.1. Analysis by Year of Establishment
- 8.2.3.2. Analysis by Company Size
- 8.2.3.3. Analysis by Location of Headquarters (Continent-wise)
- 8.2.3.4. Analysis by Location of Headquarters (Country-wise)
- 8.2.4. Technology Platform: Profiles
- 8.2.4.1. Conjugated Delivery Technologies
- 8.2.4.1.1. GalNAc Conjugate Delivery System, Alnylam Pharmaceuticals
- 8.2.4.1.1.1. Technology Overview
- 8.2.4.1.1.2. Pipeline Molecules in Development
- 8.2.4.1.1.3. Analyst's Perspective
- 8.2.4.1.2. Protein Nanoparticle (PNP) Delivery Technology, Ariz Precision Medicine
- 8.2.4.1.2.1. Technology Overview
- 8.2.4.1.2.2. Pipeline Molecules in Development
- 8.2.4.1.2.3. Analyst's Perspective
- 8.2.4.1.3 Targeted RNAi Molecule (TRiM) Platform, Arrowhead Pharmaceuticals
- 8.2.4.1.3.1. Technology Overview
- 8.2.4.1.3.2. Pipeline Molecules in Development
- 8.2.4.1.3.3. Analyst's Perspective
- 8.2.4.1.1. GalNAc Conjugate Delivery System, Alnylam Pharmaceuticals
- 8.2.4.2. Drug Discovery and Development Technologies
- 8.2.4.2.1. GalXC Conjugated RNAi Technology Platform, Dicerna Pharmaceuticals
- 8.2.4.2.1.1. Technology Overview
- 8.2.4.2.1.2. Pipeline Molecules in Development
- 8.2.4.2.1.3. Analyst's Perspective
- 8.2.4.2.2. The Vigil Platform, Gradalis
- 8.2.4.2.2.1. Technology Overview
- 8.2.4.2.2.2. Pipeline Molecules in Development
- 8.2.4.2.2.3. Analyst's Perspective
- 8.2.4.2.3. mRNAi GOLD Platform, Silence Therapeutics
- 8.2.4.2.3.1. Technology Overview
- 8.2.4.2.3.2. Pipeline Molecules in Development
- 8.2.4.2.3.3. Analyst's Perspective
- 8.2.4.2.1. GalXC Conjugated RNAi Technology Platform, Dicerna Pharmaceuticals
- 8.2.4.1. Conjugated Delivery Technologies
- 8.2.1. RNAi Triggers
9. TECHNOLOGY COMPETITIVENESS ANALYSIS
- 9.1. Chapter Overview
- 9.2. Assumptions / Key Parameters
- 9.3. Methodology
- 9.4. Technology Competitiveness Analysis: RNAi Therapeutics Technologies
- 9.4.1. Technology Competitiveness Analysis: Drug Delivery Technologies
- 9.4.2. Technology Competitiveness Analysis: Drug Discovery / Development Technologies
10. KEY THERAPEUTIC INDICATIONS
- 10.1. Chapter Overview
- 10.2. Oncological Disorders
- 10.2.1. Analysis by Target Indication and Phase of Development
- 10.2.2. Analysis by Type of RNAi Molecule
- 10.3. Infectious Diseases
- 10.3.1. Analysis by Target Indication and Phase of Development
- 10.3.2. Analysis by Type of RNAi Molecule
- 10.4. Metabolic Disorders
- 10.4.1. Analysis by Target Indication and Phase of Development
- 10.4.2. Analysis by Type of RNAi Molecule
- 10.5. Ophthalmic Diseases
- 10.5.1. Analysis by Target Indication and Phase of Development
- 10.5.2. Analysis by Type of RNAi Molecule
- 10.6. Genetic Disorders
- 10.6.1. Analysis by Target Indication and Phase of Development
- 10.6.2. Analysis by Type of RNAi Molecule
11. CLINICAL TRIAL ANALYSIS
- 11.1. Chapter Overview
- 11.2. Scope and Methodology
- 11.3. RNAi Therapeutics: Clinical Trial Analysis
- 11.3.1. Analysis by Trial Registration Year
- 11.3.2. Analysis by Trial Phase
- 11.3.3. Analysis by Trial Recruitment Status
- 11.3.4. Analysis by Type of Sponsor / Collaborator
- 11.3.5. Analysis by Type of RNAi Molecule and Trial Recruitment Status
- 11.3.6. Analysis by Therapeutic Area
- 11.3.7. Geographical Analysis by Number of Clinical Trials
- 11.3.8. Geographical Analysis by Number of Clinical Trials, Trial Phase and Recruitment Status
- 11.3.9. Geographical Analysis by Number of Clinical Trials and Type of RNAi Molecule
- 11.3.10. Geographical Analysis by Number of Clinical Trials, Type of RNAi Molecule and Trial Phase
- 11.3.11. Geographical Analysis by Number of Clinical Trials and Therapeutic Area
- 11.3.12. Geographical Analysis by Number of Clinical Trials, Therapeutic Area and Trial Phase
- 11.3.13. Geographical Analysis of Enrolled Patient Population by Location of Trial
- 11.3.14. Geographical Analysis of Enrolled Patient Population by Trial Phase and Trial Recruitment Status
- 11.3.15. Geographical Analysis of Enrolled Patient Population by Type of RNAi Molecule and Location of Trial
- 11.3.16. Geographical Analysis of Enrolled Patient Population by Type of RNAi Molecule, Trial Phase and Location of Trial
- 11.3.17. Geographical Analysis of Enrolled Patient Population by Therapeutic Area and Location of Trial
- 11.3.18. Geographical Analysis of Enrolled Patient Population by Therapeutic Area. Trial Phase and Location of Trial
- 11.4. Concluding Remarks
- 11.4.1. Key Therapeutic Candidates
- 11.4.2. Key Clinical Trials
12. KEY OPINION LEADERS
- 12.1. Chapter Overview
- 12.2. RNAi Therapeutics: Key Opinion Leaders
- 12.2.1. Analysis by Type of Organization
- 12.2.2. Analysis by Qualification
- 12.2.3. Analysis by Geographical Location of KOLs
- 12.2.4. Most Prominent Organizations: Analysis by Number of KOLs
- 12.2.5. KOL Activeness v/s KOL Strength
- 12.2.6. Most Prominent KOLs: Analysis by KOL Strength
- 12.2.7. Most Prominent KOLs: Comparison of RA Score and Third-Party Score
13. PATENT ANALYSIS
- 13.1. Chapter Overview
- 13.2. Scope and Methodology
- 13.3. RNAi Therapeutics: Patent Analysis
- 13.3.1. Analysis by Publication Year
- 13.3.2. Analysis by Patent Type and Publication Year
- 13.3.3. Analysis by CPC Code
- 13.3.4. Analysis by Type of Applicant
- 13.3.5. Analysis by Geography
- 13.3.5.1. Analysis by Geography: North American Scenario
- 13.3.5.2. Analysis by Geography: European Scenario
- 13.3.5.3. Analysis by Geography: Asia-Pacific Scenario
- 13.3.6. Emerging Focus Areas
- 13.3.7. Leading Players: Analysis by Number of Patents
- 13.4. RNAi Therapeutics: Patent Benchmarking Analysis
- 13.4.1. Analysis by Key Patent Characteristics
- 13.4.1.1. Arrowhead Pharmaceuticals and Sirna Therapeutics
- 13.4.1.2. Other Leading Patent Assignees
- 13.4.1. Analysis by Key Patent Characteristics
- 13.5. RNAi Therapeutics: Patent Valuation Analysis
14. PUBLICATION ANALYSIS
- 14.1. Chapter Overview
- 14.2. Scope and Methodology
- 14.3. Analysis by Year of Publication
- 14.4. Analysis by Type of Publication
- 14.5. Most Popular Keywords
- 14.6. Most Popular Journals: Analysis by Number of Publications
- 14.7. Most Popular Publisher: Analysis by Number of Publications
- 14.8. Most Popular Copyright Holders: Analysis by Number of Publications
- 14.9. Key Funding Institutes: Analysis by Number of Publications
15. PARTNERSHIPS AND COLLABORATIONS
- 15.1. Chapter Overview
- 15.2. Partnership Models
- 15.3. RNAi Therapeutics: Recent Partnerships and Collaborations
- 15.3.1. Analysis by Year of Partnership
- 15.3.2. Analysis by Type of Partnership
- 15.3.3. Analysis by Type of RNAi Molecule
- 15.3.4. Analysis by Scale of Partnership
- 15.3.5. Analysis by Therapeutic Area
- 15.3.6. Most Active Players: Analysis by Number of Partnerships
- 15.3.7. Regional Analysis
- 15.3.7.1. Country-wise Distribution
- 15.3.7.2. Intercontinental and Intracontinental Deals
16. FUNDING AND INVESTMENT ANALYSIS
- 16.1. Chapter Overview
- 16.2. Types of Funding
- 16.3. RNAi Therapeutics: Funding and Investment Analysis
- 16.3.1. Analysis by Cumulative Funding Instances, 2014-2021
- 16.3.2. Analysis by Amount Invested
- 16.3.3. Analysis by Type of Funding
- 16.3.4. Analysis by Year and Type of Funding
- 16.3.5. Analysis of Amount Invested Across Different Types of RNAi Molecules
- 16.3.6. Regional Analysis by Amount Invested
- 16.3.7. Most Active Players
- 16.3.8. Key Investors
- 16.4. Concluding Remarks
17. KEY COMMERCIALIZATION STRATEGIES
- 17.1. Chapter Overview
- 17.2. Successful Drug Launch Strategy: ROOTS Framework
- 17.3. Successful Drug Launch Strategy: Product Differentiation
- 17.4. Commonly Adopted Commercialization Strategies based on Stage of Development of the Product
- 17.5. Approved RNAi Therapeutics
- 17.6. Key Commercialization Strategies Adopted by RNAi-based Therapy Developers
- 17.6.1. Strategies Adopted Before Therapy Approval
- 17.6.1.1. Participation in Global Events
- 17.6.1.1.1. Onpattro
- 17.6.1.1.2. Givlaari
- 17.6.1.1.3. Oxlumo
- 17.6.1.2. Collaboration with Stakeholders and Pharmaceutical Firms
- 17.6.1.2.1. Leqvio
- 17.6.1.3. Indication Expansion
- 17.6.1.3.1. Onpattro
- 17.6.1.3.2. Givlaari
- 17.6.1.3.3. Oxlumo
- 17.6.1.3.4. Leqvio
- 17.6.1.1. Participation in Global Events
- 17.6.2. Strategies Adopted During / Post Therapy Approval
- 17.6.2.1. Participation in Global Events
- 17.6.2.1.1. Onpattro
- 17.6.2.1.2. Leqvio
- 17.6.2.2. Geographical Expansion
- 17.6.2.2.1. Onpattro
- 17.6.2.2.2. Givlaari
- 17.6.2.2.3. Oxlumo
- 17.6.2.2.4. Leqvio
- 17.6.2.3. Patient Assistance Programs
- 17.6.2.3.1. Onpattro
- 17.6.2.3.2. Givlaari
- 17.6.2.3.3. Oxlumo
- 17.6.2.4. Awareness Through Product Websites
- 17.6.2.5. Collaboration with Stakeholders and Pharmaceutical Firms
- 17.6.2.5.1. Onpattro
- 17.6.2.5.2. Givlaari
- 17.6.2.5.3. Oxlumo
- 17.6.2.5.4. Leqvio
- 17.6.2.6. Aligning with Government Initiatives to Ensure High Adoption Rate
- 17.6.2.6.1. Onpattro
- 17.6.2.6.2. Givlaari
- 17.6.2.6.3. Leqvio
- 17.6.2.1. Participation in Global Events
- 17.6.1. Strategies Adopted Before Therapy Approval
- 17.7. Concluding Remarks
18. RNAi IN DIAGNOSTICS
- 18.1. Chapter Overview
- 18.2. Key Characteristics of Biomarkers
- 18.3. Circulating miRNA Biomarkers
- 18.4. miRNA Biomarkers in Oncological Disorders
- 18.4.1. Importance of Early Cancer Detection
- 18.4.2. Cancer Screening and Diagnosis
- 18.4.3. Conventional Cancer Diagnostics
- 18.4.3.1. Biopsy
- 18.4.4. Need for Non-Invasive Approaches
- 18.4.5. Key Indications
- 18.4.5.1. Breast Cancer
- 18.4.5.2. Colorectal Cancer
- 18.4.5.3. Gastric Cancer
- 18.4.5.4. Hematological Cancer
- 18.4.5.4.1. Acute Myeloid Leukemia
- 18.4.5.4.2 Lymphoma
- 18.4.5.5. Lung Cancer
- 18.4.5.6. Prostate Cancer
- 18.5. miRNA Biomarkers in Cardiovascular Diseases
- 18.5.1. Key Indications
- 18.5.1.1. Coronary Artery Disease
- 18.5.1.2. Myocardial Infarction
- 18.5.1. Key Indications
- 18.6. miRNA Based Diagnostic Tests
19. RNAi THERAPEUTICS SERVICE PROVIDERS
- 19.1. Chapter Overview
- 19.2. Analysis by Types of Service Providers
- 19.3. RNAi Therapeutics: List of CROs
- 19.3.1. Analysis by Year of Establishment
- 19.3.2. Analysis by Location of Headquarters
- 19.3.3. Analysis by Company Size
- 19.3.4. Analysis by Type of RNAi Molecule
- 19.4. RNAi Therapeutics: List of CMOs
- 19.4.1. Analysis by Year of Establishment
- 19.4.2. Analysis by Location of Headquarters
- 19.4.3. Analysis by Company Size
- 19.4.4. Analysis by Type of RNAi Molecule
- 19.5. RNAi Therapeutics: List of Consumables and Other Service Providers
- 19.5.1. Analysis by Year of Establishment
- 19.5.2. Analysis by Location of Headquarters
- 19.5.3. Analysis by Company Size
- 19.5.4. Analysis by Type of RNAi Molecule
20. DEMAND ANALYSIS
- 20.1. Chapter Overview
- 20.2. Methodology
- 20.3. Global Demand for RNAi Therapeutics, 2022-2035
- 20.3.1. Analysis by Therapeutic Approach
- 20.3.2. Analysis by Target Therapeutic Area
- 20.3.3. Analysis by Route of Administration
- 20.3.4. Analysis by Key Players
- 20.3.5. Analysis by Geography
21. MARKET SIZING AND OPPORTUNITY ANALYSIS
- 21.1. Chapter Overview
- 21.2. Scope and Limitations
- 21.3. Key Assumptions and Forecast Methodology
- 21.4. Overall RNAi Therapeutics Market, 2022-2035
- 21.4.1. RNAi Therapeutics Market: Analysis by Type of RNAi Molecule
- 21.4.2. RNAi Therapeutics Market: Analysis by Therapeutic Area
- 21.4.3. RNAi Therapeutics Market: Analysis by Route of Administration
- 21.4.4. RNAi Therapeutics Market: Share of Leading Players
- 21.4.5. RNAi Therapeutics Market: Analysis by Geography
- 21.5. RNAi Therapeutics Market: Value Creation Analysis
- 21.6. RNAi Therapeutics Market: Product-wise Sales Forecasts
- 21.6.1. Onpattro(R)
- 21.6.1.1. Target Patient Population
- 21.6.1.2. Sales Forecast
- 21.6.1.3. Net Present Value
- 21.6.1.4. Value Creation Analysis
- 21.6.2. Givlaari(TM)
- 21.6.2.1. Target Patient Population
- 21.6.2.2. Sales Forecast
- 21.6.2.3. Net Present Value
- 21.6.2.4. Value Creation Analysis
- 21.6.3. Leqvio(R)
- 21.6.3.1. Target Patient Population
- 21.6.3.2. Sales Forecast
- 21.6.3.3. Net Present Value
- 21.6.3.4. Value Creation Analysis
- 21.6.4. Oxlumo(TM)
- 21.6.4.1. Target Patient Population
- 21.6.4.2. Sales Forecast
- 21.6.4.3. Net Present Value
- 21.6.4.4. Value Creation Analysis
- 21.6.5. Fitusiran
- 21.6.5.1. Target Patient Population
- 21.6.5.2. Sales Forecast
- 21.6.5.3. Net Present Value
- 21.6.5.4. Value Creation Analysis
- 21.6.6. Vutrisiran
- 21.6.6.1. Target Patient Population
- 21.6.6.2. Sales Forecast
- 21.6.6.3. Net Present Value
- 21.6.6.4. Value Creation Analysis
- 21.6.7. SYL-1001
- 21.6.7.1. Target Patient Population
- 21.6.7.2. Sales Forecast
- 21.6.7.3. Net Present Value
- 21.6.7.4. Value Creation Analysis
- 21.6.8. Vigil-EWS
- 21.6.8.1. Target Patient Population
- 21.6.8.2. Sales Forecast
- 21.6.8.3. Net Present Value
- 21.6.8.4. Value Creation Analysis
- 21.6.9. Nedosiran
- 21.6.9.1. Target Patient Population
- 21.6.9.2. Sales Forecast
- 21.6.9.3. Net Present Value
- 21.6.9.4. Value Creation Analysis
- 21.6.1. Onpattro(R)
22. SWOT ANALYSIS
- 22.1. Chapter Overview
- 22.2. Strengths
- 22.3. Weaknesses
- 22.4. Opportunities
- 22.5. Threats
- 22.6. Comparison of SWOT Factors
- 22.6.1 Concluding Remarks
23. INTERVIEW TRANSCRIPT(S)
24. CONCLUSION
- 24.1. Chapter Overview
- 24.2. Key Takeaways



















