
|
시장보고서
상품코드
1737059
임상시험 소프트웨어 시장 : 배포 유형별, 제공 유형별, 소프트웨어 기능별, 최종 사용자별, 지역별Clinical Trial Software Market Distribution by Type of Deployment, Type of Delivery, Features of Software and Geographical Regions |
||||||
세계의 임상시험 소프트웨어 시장 규모는 2035년까지 예측 기간 동안 14%의 연평균 복합 성장률(CAGR)을 나타내며 현재 6억 9,000만 달러에서 2035년까지 36억 8,000만 달러로 성장할 것으로 추정됩니다.
시장 세분화는 시장 규모와 시장 기회를 다음 매개 변수로 구분합니다.
배포 유형별
- 온클라우드
- 온프레미스
제공 유형별
- 웹 기반
- 원격 모니터링
소프트웨어 기능별
- EDC
- eCOA/ePRO
- eConsent
최종 사용자별
- 제약/바이오테크놀러지 산업
- 학술 및 연구기관
- 기타 산업
지역별
- 북미
- 유럽
- 아시아태평양
임상시험 소프트웨어 시장 : 성장과 동향
임상시험은 사람에 대한 의학적, 외과적, 행동학적 개입을 평가하고 질병의 진단과 예방/치료를 위한 신규 접근법을 조사하기 위해 고안된 긍정적인 생물의학 연구입니다. 정적 적응증에 대한 약물의 안전성과 효능을 검증하기 위해 매우 정확하고 정교한 임상시험 데이터가 필요합니다. 치료 개입의 개발에 종사하는 기업이 직면하는 확실한 데이터의 부족 등이 있습니다. 실제로 기존의 수작업 임상시험은 신약 개발 기간 중 거의 50%의 시간을 소비합니다. 이런 상황 속, 임상시험 소프트웨어는 실시간의 분석, 데이터 관리, 약제의 유해 영향의 추적이 가능하기 때문에 큰 주목을 모으고 있습니다.

최근의 동향에서 다양한 기업들이 EDC, eCOA/ePRO, eConsent를 포함한 많은 임상시험 소프트웨어/임상시험 관리 시스템을 개발하고 있습니다. 이러한 도구는 실시간 임상시험을 가능하게 하고 약물 검사 및 분석을 위한 환자의 규정 준수를 향상시킵니다. 그 중요성 때문에 여러 임상시험 소프트웨어 시장 기업이 현재이 분야에 진출하고 혁신적인 기술을 개발하고 있습니다. 특히, 이러한 기술은 임상시험의 결과를 촉진하고 제약 회사의 부담을 줄일 수 있습니다. 자동화된 임상시험 소프트웨어에 대한 수요가 증가함에 따라 이 시장은 예측 기간 동안 큰 성장이 예상됩니다.
임상시험 소프트웨어 시장 : 주요 인사이트
이 보고서는 임상시험 소프트웨어 시장의 현재 상태를 파악하고 업계 내 잠재적 성장 기회를 확인합니다. 주요 조사 결과는 다음과 같습니다.
- 전 세계 70개 이상의 기업들이 임상 조사 방법의 분산을 가능하게 하는 소프트웨어 솔루션을 개발하고 임상시험에 소요되는 시간과 비용을 최적화하고 있다고 주장합니다.
- 현재 시장 상황은 매우 단편화되어 있으며 고급 기능을 갖춘 임상시험 소프트웨어를 제공하는 기존 기업과 신규 진출기업이 모두 존재합니다. 실제로 2000년 이후 임상시험 소프트웨어 개발에 주력하는 신흥기업이 51개 이상 설립되었습니다.
- 경쟁 우위를 확립하기 위해 각 회사는 기존 기능을 적극적으로 확장하고, 각각의 제공 서비스를 강화하고, 진화하는 업계 벤치마크에 대응하고 있습니다.
- 유리한 수익을 기대하고 많은 공적 및 사적 투자자들이 많은 투자를 하고 있으며, 자금 조달 활동이 급증하고 있습니다. 특히 북미에서는 이 분야에서의 자금조달 사례의 약 90%가 되고 있습니다.

- 이 분야에서는 지난 몇 년간 파트너십 및 협업이 크게 증가하고 있으며 이해 관계자의 관심이 높아지고 있음을 보여줍니다.
- 주목해야할 것은 M&A 사례의 대부분이 2023년에 보고된 것으로, 서서히 통합으로 변화하고 있는 것을 나타냈습니다.
- 이 시장은 향후 10년간 연률 14%를 나타낼 것으로 예측됩니다.

임상시험 소프트웨어 시장 : 주요 부문
eConsent 소프트웨어 부문이 세계의 임상시험 소프트웨어 시장에서 최대 점유율을 차지
소프트웨어 기능별로 세계의 임상시험 소프트웨어 시장은 EDC, eCOA/ePRO, eConsent 소프트웨어로 구분됩니다. 현재 임상시험 소프트웨어 시장의 대부분은 eConsent 소프트웨어가 차지하고 있습니다.
지역별로 볼 때 시장은 북미, 유럽, 아시아태평양으로 구분됩니다. 현재 시나리오에서는 북미가 최대 시장 점유율을 얻고 아시아태평양 시장은 예측 기간 동안 유리한 성장을 보일 것으로 예측됩니다.
본 보고서에서는 세계의 임상시험 소프트웨어 시장에 대해 조사했으며 시장 개요와 함께 배포 유형별, 제공 유형별, 소프트웨어 기능별, 최종 사용자별, 지역별 동향, 시장 진출기업 프로파일 등의 정보를 제공합니다.
목차
제1장 서문
제2장 주요 요약
제3장 서론
- 장 개요
- 임상 연구에 있어서 기존의 제약
- 가상 임상시험
- 가상 임상시험 관리와 관련된 기회와 과제
- 향후 전망
제4장 시장 상황 : 임상시험 소프트웨어 시장
- 장 개요
- 임상시험 소프트웨어 시장 : 제품 리스트
- 임상시험 소프트웨어 시장 : 개발자의 상황
제5장 북미의 임상시험 소프트웨어 개발 회사 : 기업 프로파일
- 장 개요
- Advarra
- Arisglobal
- AssistRx
- Clario
- IBM
- IQVIA
- Medidata
- Oracle
- Signant Health
- Veeva
제6장 유럽의 임상시험 소프트웨어 개발 기업 : 기업 프로파일
- 장 개요
- Calyx
제7장 기업 경쟁력 분석
제8장 파트너십 및 협업
제9장 합병과 인수
제10장 자금 조달과 투자 분석
제11장 시장 예측과 기회 분석
- 장 개요
- 예측 조사 방법과 주요 전제조건
- 세계의 임상시험 소프트웨어 시장(2021-2035년)
- 임상시험 소프트웨어 시장(2021-2035년) : 배포 유형별 분포
- 임상시험 소프트웨어 시장(2021-2035년) : 제공 유형별 분포
- 임상시험 소프트웨어 시장(2021-2035년) : 소프트웨어 기능별 분포
- 임상시험 소프트웨어 시장(2021-2035년) : 지역별 분포
제12장 결론
제13장 부록 1 : 표 형식의 데이터
제14장 부록 2 : 기업 및 단체 일람
KTH 25.06.10CLINICAL TRIAL SOFTWARE MARKET: OVERVIEW
As per Roots Analysis, the global clinical trial software market is estimated to grow from USD 0.69 billion in the current year to USD 3.68 billion by 2035, at a CAGR of 14% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Deployment
- On-Cloud
- On-Premises
Type of Delivery
- Web-Based
- Remote Monitoring
Features of Software
- EDC
- eCOA/ePRO
- eConsent
End Users
- Pharmaceutical / Biotechnology Industries
- Academic and Research Institutes
- Other Industries
Geographical Regions
- North America
- Europe
- Asia-Pacific
CLINICAL TRIAL SOFTWARE MARKET: GROWTH AND TRENDS
Clinical trials are prospective biomedical research studies designed to evaluate medical, surgical or behavioral interventions in people and investigate novel approaches for the diagnosis and prevention / treatment of diseases. In order to gain marketing approval from regulatory authorities for a novel therapeutic intervention, highly accurate and elaborate clinical trial data is required to validate the drug's safety and effectiveness towards a specific target indication. However, there are several challenges associated with this traditional way of clinical research. Some of these include high capital investment, low patient recruitment rates and lack of robust data faced by companies involved in the development of various therapeutic interventions. This leads to inefficiency in generating sufficient clinical evidence, resulting in massive capital losses for drug developers, as well as patients accessing these life-saving therapies. In fact, manual conventional clinical trials consume almost 50% of the time during drug development. In this context, clinical trial software has gained significant attention owing to its ability for real-time analysis, data management, and tracking of the adverse impact of drugs.
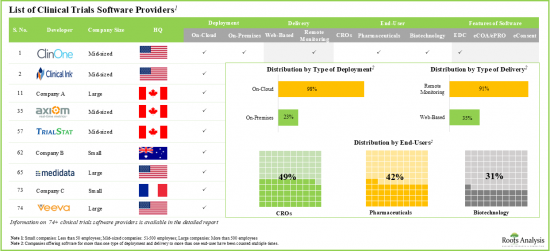
In recent years, various players have developed a number of clinical trial software / clinical trial management systems, including EDC, eCOA / ePRO, and eConsent. These tools enable real-time clinical studies and improve patient compliance for drug testing and analysis. Owing to its significance, several clinical trial software market players are currently engaged in this field to develop innovative technologies. Notably, these technologies encourage successful outcomes of clinical trials and reduce the burden of pharmaceutical companies. Given the increasing demand for automated clinical trial software, the market is expected to witness substantial growth during the forecast period.
CLINICAL TRIAL SOFTWARE MARKET: KEY INSIGHTS
The report delves into the current state of the clinical trial software market and identifies potential growth opportunities within the industry. Some key findings from the report include:
- 70+ companies worldwide claim to have developed software solutions allowing decentralization of the clinical research process, optimizing time and cost spent on clinical trials.
- The current market landscape is highly fragmented, with the presence of both established players and new entrants offering clinical trials software with advanced features. In fact, since 2000, over 51 start-ups focused on developing clinical trials software have been established.
- In pursuit of building a competitive edge, companies are actively expanding their existing capabilities to enhance their respective offerings and comply with evolving industry benchmarks.
- Foreseeing lucrative returns, many public and private investors have significant investments, marking a surge in funding activity. Notably, North America witnessed around 90% of funding instances in this domain.
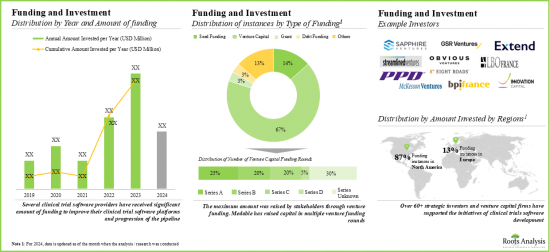
- The domain has witnessed a considerable increase in partnerships and collaborations over the past few years, indicating the rising interest of stakeholders.
- Notably, most of the M&A instances were reported in 2023, indicating a gradual shift towards consolidation. Further, majority (87%) of agreements were acquisitions, followed by mergers.
- We expect the market to grow at an annualized rate of 14% in the coming decade; the opportunity is likely to be well distributed across type of deployment, features of software, type of delivery and geographical regions.
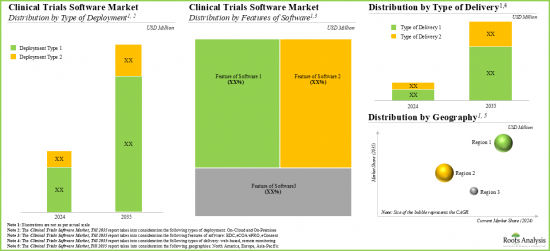
CLINICAL TRIAL SOFTWARE MARKET: KEY SEGMENTS
The eConsent Software Segment Holds the Largest Share of the Global Clinical Trial Software Market
Based on the features of software, the global market for clinical trial software is segmented into EDC, eCOA/ePRO and eConsent software. Currently, the majority of the clinical trial software market is captured by eConsent software. It is worth highlighting that the global clinical trial software market for eCOA / ePRO segment is likely to grow at a relatively higher CAGR.
North America Accounts for the Largest Share of the Market
Based on the geographical regions, the market is segmented into North America, Europe and Asia-Pacific. In the current scenario, North America is likely to capture the largest market share while the market in Asia-Pacific is anticipated to demonstrate lucrative growth during the forecast period.
Example Players in the Clinical Trial Software Market
- Advarra
- Arisglobal
- AssistRx
- Calyx
- Clario
- IBM
- IQVIA
- Medidata
- Oracle
- Signant Health
- Veeva
CLINICAL TRIAL SOFTWARE MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global clinical trial software market, focusing on key market segments, including [A] type of deployment, [B] type of delivery, [C] features of software, [D] end users and [E] geographical regions.
- Clinical Trials Software Market Landscape: A comprehensive evaluation of clinical trials software market, based on several relevant parameters, such as [A] type of deployment, [B] type of delivery, [C] type of end-user, [D] features of software, [E] trial design and [F] type of technology. Additionally, a comprehensive evaluation of the companies engaged in developing clinical trials software, based on several relevant parameters, such as [G] year of establishment, [H] company and [I] location of headquarters.
- Company Profiles: In-depth profiles of key players engaged in the development of clinical trials software, focusing on [A] overview of the company, [B] product portfolio, and [C] recent developments and [D] an informed future outlook.
- Company Competitiveness Analysis: A comprehensive competitive analysis of clinical trials software developers, examining factors, such as [A] supplier strength, [B] product portfolio strength and [C] service applicability.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the global clinical trials software market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] geographical distribution of partnership activity and [D] most active players (in terms of the number of partnerships signed).
- Mergers and Acquisitions: An in-depth analysis of the mergers and acquisitions undertaken in this domain, based on relevant parameters, such as [A] type of agreement, [B] year of mergers and acquisitions, [C] geographical location and [D] most active players (in terms of the number of mergers and acquisitions).
- Funding and Investment Analysis: An in-depth analysis of the fundings raised by companies engaged in this domain, based on relevant parameters, such as [A] year of funding, [B] type of funding, [C] amount invested, [D] most active players and [F] most active investors.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Existing Constraints in Clinical Research
- 3.2.1. Increasing Trial Costs and Complexity
- 3.2.2. Evolving Regulatory Standards
- 3.2.3. Patient Recruitment and Retention-Related Challenges
- 3.2.4. Inefficient Data Handling
- 3.3. Virtual Clinical Trials
- 3.3.1. Electronic Data Capture Solutions
- 3.3.2. Electronic Clinical Outcome Assessment and Electronic Patient Reported Outcome Solutions (eCOA / ePRO)
- 3.3.3. Electronic Consent Solutions
- 3.4. Opportunities and challenges associated with Virtual Clinical Trials Management
- 3.5 Future Perspectives
4 MARKET LANDSCAPE: CLINICAL TRIALS SOFTWARE MARKET
- 4.1. Chapter Overview
- 4.2. Clinical Trials Software Market: List of Products
- 4.2.1. Analysis by Type of Deployment
- 4.2.2. Analysis by Type of Delivery
- 4.2.3. Analysis by End-User
- 4.2.4. Analysis by Features of Software
- 4.2.5. Analysis by Trial Design
- 4.2.6. Analysis by Type of Technology
- 4.3. Clinical Trials Software Market: Developer Landscape
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Geography
5 CLINICAL TRIALS SOFTWARE DEVELOPERS IN NORTH AMERICA: COMPANY PROFILES
- 5.1. Chapter Overview
- 5.2. Advarra
- 5.2.1. Company Overview
- 5.2.2. Product Portfolio: Clinical Trials Software
- 5.2.3. Recent Developments and Future Outlook
- 5.3. Arisglobal
- 5.3.1. Company Overview
- 5.3.2. Product Portfolio: Clinical Trials Software
- 5.3.3. Recent Developments and Future Outlook
- 5.4. AssistRx
- 5.4.1. Company Overview
- 5.4.2. Product Portfolio: Clinical Trials Software
- 5.4.3. Recent Developments and Future Outlook
- 5.5. Clario
- 5.5.1. Company Overview
- 5.5.2. Product Portfolio: Clinical Trials Software
- 5.5.3. Recent Developments and Future Outlook
- 5.6. IBM
- 5.6.1. Company Overview
- 5.6.2. Product Portfolio: Clinical Trials Software
- 5.6.3. Recent Developments and Future Outlook
- 5.7. IQVIA
- 5.7.1. Company Overview
- 5.7.2. Product Portfolio: Clinical Trials Software
- 5.7.3. Recent Developments and Future Outlook
- 5.8. Medidata
- 5.8.1. Company Overview
- 5.8.2. Product Portfolio: Clinical Trials Software
- 5.8.3. Recent Developments and Future Outlook
- 5.9. Oracle
- 5.9.1. Company Overview
- 5.9.2. Product Portfolio: Clinical Trials Software
- 5.9.3. Recent Developments and Future Outlook
- 5.10. Signant Health
- 5.10.1. Company Overview
- 5.10.2. Product Portfolio: Clinical Trials Software
- 5.10.3. Recent Developments and Future Outlook
- 5.11. Veeva
- 5.11.1. Company Overview
- 5.11.2. Product Portfolio: Clinical Trials Software
- 5.11.3. Recent Developments and Future Outlook
6 CLINICAL TRIALS SOFTWARE DEVELOPERS IN EUROPE: COMPANY PROFILES
- 6.1. Chapter overview
- 6.2. Calyx
- 6.2.1. Company Overview
- 6.2.2. Product Portfolio: Clinical Trials Software
- 6.2.3. Recent Developments and Future Outlook
7 COMPANY COMPETITIVENESS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Key Parameters and Methodology
- 7.3. Competitiveness Analysis: Companies providing clinical trials software developers
- 7.4. Competitiveness Analysis: Companies providing clinical trials software in North America
- 7.5. Competitiveness Analysis: Companies providing clinical trials software in Europe
- 7.6. Competitiveness Analysis: Companies providing clinical trials software in Asia-Pacific
8 Partnerships and Collaborations
- 8.1. Chapter Overview
- 8.2. Partnership Models
- 8.3. Clinical Trials Software Market: Partnerships and Collaborations
- 8.3.1. Analysis by Year of Partnership
- 8.3.2. Analysis by Type of Partnership
- 8.3.3. Analysis by Type and Year of Partnership
- 8.4. Geographical Analysis
- 8.4.1. Analysis by Intracontinental and Intercontinental Agreements
- 8.4.2. Analysis by Local and International Agreements
- 8.5. Most Active Players: Analysis by Number of Partnerships
9 MERGERS AND ACQUISITIONS
- 9.1. Chapter Overview
- 9.2. Mergers and Acquisitions Models
- 9.3. Clinical Trials Software Market: Mergers and Acquisitions
- 9.3.1. Analysis by Type of Agreement
- 9.3.2. Analysis by Year of Mergers and Acquisitions
- 9.4. Analysis by Geographical Activity
- 9.4.1. Region-wise Analysis
- 9.4.2. Mergers and Acquisitions: Intercontinental and Intracontinental Deals
- 9.5. Most Active Players: Analysis by Number of Instances Acquisitions and Mergers
10 FUNDING AND INVESTMENT ANALYSIS
- 10.1. Chapter Overview
- 10.2. Types of Funding Instances
- 10.3. Clinical Trials Software Market: Recent Funding Instances
- 10.3.1. Analysis by Year of Investment
- 10.3.2. Analysis by Amount Invested
- 10.3.3. Analysis by Type of Funding
- 10.4. Most Active Players: Analysis by Number of Funding Instances
- 10.5. Regional Analysis by Amount Invested
- 10.6. Concluding Remarks
11 MARKET FORECAST AND OPPORTUNITY ANALYSIS
- 11.1. Chapter Overview
- 11.2. Forecast Methodology and Key Assumptions
- 11.3. Global Clinical Trials Software Market, 2021-2035
- 11.3.1. Clinical Trials Software Market, 2021-2035: Distribution by Type of Deployment
- 11.3.2. Clinical Trials Software Market, 2021-2035: Distribution by Type of Delivery
- 11.3.3. Clinical Trials Software Market, 2021-2035: Distribution by Features of Software
- 11.3.4. Clinical Trials Software Market, 2021-2035: Distribution by Geographical Region
- 11.3.4.1. Clinical Trials Software Market in North America, 2021-2035
- 11.3.4.1.1. Clinical Trials Software Market in North America, 2021-2035: Distribution by Type of Deployment
- 11.3.4.1.2. Clinical Trials Software Market in North America, 2021-2035: Distribution by Type of Delivery
- 11.3.4.1.3. Clinical Trials Software Market in North America, 2021-2035: Distribution by Features of Software
- 11.3.4.2. Clinical Trials Software Market in Europe, 2021-2035
- 11.3.4.2.1. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Type of Deployment
- 11.3.4.2.2. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Type of Delivery
- 11.3.4.2.3. Clinical Trials Software Market in Europe, 2021-2035: Distribution by Features of Software
- 11.3.4.3. Clinical Trials Software Market in Asia-Pacific, 2021-2035
- 11.3.4.3.1. Clinical Trials Software Market in Asia-Pacific, 2021-2035: Distribution by Type of Deployment
- 11.3.4.3.2. Clinical Trials Software Market Asia-Pacific, 2021-2035: Distribution by Type of Delivery
- 11.3.4.3.3. Clinical Trials Software Market Asia-Pacific, 2021-2035: Distribution by Features of Software
- 11.3.4.1. Clinical Trials Software Market in North America, 2021-2035
12. CONCLUSION
- 12.1. Chapter Overview



















