
|
시장보고서
상품코드
1762539
비바이러스성 트랜스펙션 시약 시장 : 업계 동향과 세계 예측 - 비바이러스성 트랜스펙션법 유형별, 용도별, 최종사용자별, 주요 지역별Non-Viral Transfection Reagents Market : Industry Trends and Global Forecasts - Distribution by Type of Non-Viral Transfection Method, Application Areas, End-User, and Key Geographical Regions |
||||||
비바이러스성 트랜스펙션 시약 시장 : 개요
세계의 비바이러스성 트랜스펙션 시약 시장 규모는 올해, 6억 3,300만 달러에 달했습니다. 이 시장은 2035년까지의 예측 기간 중 8.0%의 CAGR로 확대할 것으로 예측됩니다.
시장 세분화에서는 시장 규모 및 기회 분석을 다음과 같은 매개 변수로 구분합니다.
비바이러스성 형질전환법 유형
- 화학적 방법
- 물리적 방법
- 다른 방법
용도
- 임상
- 연구
최종사용자
- 학술-연구기관
- 제약회사
- 기타
주요 지역
- 북미
- 유럽
- 아시아태평양
- 기타 지역
비바이러스성 전사 시약 시장 : 성장과 동향
수년 동안 많은 핵산 기반 치료법이 규제 당국의 승인을 받았으며, 이는 치료의 가능성과 인기가 높아지고 있음을 입증합니다. 이에 따라 이러한 치료법을 효율적으로 전달할 수 있는 벡터에 대한 수요가 증가하고 있습니다. 특히 트랜스펙션은 특정 운반체/벡터를 이용하여 유전물질/핵산을 인위적으로 세포에 도입하는 과정 중 하나입니다. 그러나 바이러스 벡터를 이용한 기존의 트랜스펙션은 면역원성 및 세포독성 관련 합병증, 높은 개발 비용 등 여러 가지 문제점이 있습니다. 그 결과, 비바이러스성 형질전환 시약과 같은 대체 유전자 약물 비히클에 대한 수요가 증가하고 있습니다. 비바이러스성 전달 시약은 바이러스성 벡터와 관련된 염증 및 비특이적 도입과 같은 한계를 극복할 수 있습니다. 또한 이러한 벡터는 바이러스 기반 벡터보다 훨씬 비용 효율적이며, 유전자 치료제 시장에서 보다 저렴한 제품 개발에 사용될 수 있는 가능성을 시사하고 있습니다.
비바이러스성 형질전환법에서 세포로의 유전자 도입은 숙주세포에 DNA를 직접 도입하는 방법과 지질, 고분자, 리포폴리머와의 복합체 형성을 통해 캐리어 유전자를 도입하는 방법이 있습니다. 두 경우 모두 유전자 서열은 플라스미드(벡터)에 삽입되어 숙주 내에서 발현됩니다. 면역원성이 낮기 때문에 비바이러스성 전달 시스템은 합병증의 위험 없이 유전물질을 재투여할 수 있습니다. 또한 이러한 시약은 대량 생산이 용이하여 바이러스성 벡터보다 경제적입니다.
비바이러스성 트랜스펙션 시약 시장 : 주요 인사이트
이 보고서는 비바이러스성 트랜스펙션 시약 시장의 현황을 조사하고 업계내 잠재적인 성장 기회를 파악합니다. 주요 조사 결과는 다음과 같습니다.
- 현재 시중에는 185개 이상의 비바이러스성 유전자 전달 시약이 판매되고 있으며, 이 중 60%는 지질 기반 캐리어를 사용하여 유전 물질의 전달을 가능하게 합니다.
- 경쟁 우위를 구축하기 위해 업계 이해관계자들은 진화하는 업계 벤치마크에 맞추어 각 제품을 적극적으로 강화하고 있습니다.
- DNA, RNA, 단백질 단편을 다양한 유형의 세포에 도입하기 위해 20가지 이상의 일렉트로포레이션 기반 시스템과 35가지 이상의 기타 비바이러스성 트랜스펙션 시스템이 개발되고 있습니다.
- 신흥 시장은 시장 경쟁력을 유지하기 위해 각 시스템에 고급 기능을 통합하는 데 주력하고 있습니다.
- 현재 비바이러스성 전사 및 시약 개발 기업 시장 상황은 전 세계 주요 지역에 기존 기업과 신생 기업이 모두 존재한다는 특징이 있습니다.
- 최근 수년간, 비바이러스성 이식 방법 및 기술과 관련된 과학 문헌의 출판이 눈에 띄게 증가했습니다.
- 지난 4년간 비바이러스성 형질전환법 관련 특허가 약 870건 출원/취득되었으며, 이 중 60% 이상이 비학술적 기업에 의해 출원/취득되었습니다.
- 대형 제약사들도 유리한 가능성을 내다보고 비바이러스성 이식에 초점을 맞춘 몇 가지 노력을 기울이고 있습니다. 특히 지난 2년 동안 이들 기업이 체결한 공동연구는 모두 전임상 업무에 초점을 맞춘 것이었습니다.
- 혁신가들은 첨단 치료제의 가격을 최적화하기 위해 비바이러스성 전달 시약 및 시스템 개발 기업과 제휴를 맺을 것으로 예측됩니다.
- 비용은 비 바이러스성 트랜스펙션 기술 채택에 있으며, 중요한 결정 요인입니다. 가격 전략의 프레임워크는 시약의 경쟁력 있는 가격을 평가하는 데 있으며, 진입기업에 도움이 될 것으로 보입니다.
- 이 시장은 연평균 8.0%의 성장률을 보일 것으로 예측됩니다. 예측된 기회는 트랜스펙션 방법, 용도, 최종사용자, 주요 지역별로 잘 분산될 것으로 예측됩니다.
비바이러스성 전사 시약 시장 : 주요 부문별 시장 현황
비바이러스성 형질전환법 유형에 따라 시장은 화학적 방법, 물리적 방법, 기타 방법으로 구분됩니다. 현재 전 세계 비바이러스성 트랜스펙션 시약 시장에서 물리적 방법이 가장 큰 점유율을 차지하고 있습니다. 또한 기타 방법 부문의 비바이러스성 전사 시약 시장은 예측 기간 중 가장 높은 시장 성장성을 보일 것으로 예측됩니다.
용도별로 시장은 임상 응용과 연구 응용으로 구분됩니다. 현재, 연구 용도는 전 세계 비바이러스성 트랜스펙션 시약 시장에서 가장 큰 점유율을 차지하고 있습니다. 또한 임상 용도 시장은 예측 기간 중 더 높은 CAGR로 성장할 것으로 예측됩니다.
시장은 최종사용자별로 학술 및 연구기관, 제약회사, 기타 최종사용자로 구분됩니다. 현재 전 세계 비바이러스성 트랜스펙션 시약 시장에서 가장 큰 비중을 차지하는 것은 학술 및 연구 기관입니다. 그러나 제약 회사 부문은 예측 기간 중 더 높은 CAGR로 성장할 것으로 예측됩니다.
주요 지역별로 시장은 북미, 유럽, 아시아태평양 및 기타 지역으로 분류됩니다. 현재 북미는 전 세계 비바이러스성 트랜스펙션 시약 시장을 독점하고 있으며, 가장 큰 매출 점유율을 차지하고 있습니다. 또한 아시아태평양 시장은 향후 더 높은 CAGR로 성장할 가능성이 높습니다.
비바이러스성 트랜스펙션 시약 시장의 참여 기업 예
- Altogen Biosystems
- Bio-Rad Laboratories
- BEX
- BTX
- Celsion
- Genprex
- Inovio Pharmaceuticals
- MaxCyte
- MilliporeSigma
- Nepa Gene
- OZ Biosciences
- Thermo Fisher Scientific
목차
제1장 서문
제2장 개요
제3장 서론
- 챕터 개요
- 트랜스펙션 서론
- 트랜스펙션 방법
- 트랜스펙션의 응용
- 향후 전망
제4장 비바이러스성 트랜스펙션 시약 : 시장 구도
- 챕터 개요
- 비바이러스성 트랜스펙션 시약 리스트
- 비바이러스성 트랜스펙션 시약 개발자 리스트
제5장 일렉트로포레이션 기반 트랜스펙션 시스템 : 시장 구도
- 챕터 개요
- 일렉트로포레이션 기반 트랜스펙션 시스템의 리스트
- 일렉트로포레이션 기반 트랜스펙션 시스템 개발자 리스트
제6장 기타 비바이러스성 트랜스펙션 시스템 : 시장 구도
- 챕터 개요
- 기타 비바이러스성 트랜스펙션 시스템의 리스트
- 기타 비바이러스성 트랜스펙션 시스템 개발자 리스트
제7장 기업 경쟁력 분석
- 챕터 개요
- 조사 방법과 주요 파라미터
- 비바이러스성 트랜스펙션 시약 개발 기업 : 기업 경쟁력 분석
제8장 기술 경쟁력 분석
- 챕터 개요
- 조사 방법과 주요 파라미터
- 일렉트로포레이션 기반 트랜스펙션 시스템 : 기술 경쟁력 분석
- 기타 비바이러스성 트랜스펙션 시스템 : 기술 경쟁력 분석
제9장 기업 개요
- 챕터 개요
- 비바이러스성 트랜스펙션 시약 개발 회사
- MilliporeSigma
- OZ Biosciences
- Thermo Fisher Scientific
- 일렉트로포레이션 기반 트랜스펙션 시스템 개발 회사
- BEX
- Bio-Rad Laboratories
- BTX(A subsidiary of Harvard Bioscience)
- MaxCyte
- NepaGene
- 기타 비바이러스성 트랜스펙션 시스템 개발 회사
- Imunon(Formerly known as Celsion)
- Genprex
- Inovio Pharmaceuticals
제10장 잠재적 전략적 파트너
- 챕터 개요
- 범위와 조사 방법
- 비바이러스성 트랜스펙션 시스템 개발자 : 북미의 잠재적 전략적 파트너
- 비바이러스성 트랜스펙션 시스템 개발자 : 유럽의 잠재적 전략적 파트너
- 비바이러스성 트랜스펙션 시스템 개발자 : 아시아태평양 및 기타 지역의 잠재적 전략적 파트너
제11장 대형 제약회사 구상
- 챕터 개요
- 범위와 조사 방법
- 비바이러스성 트랜스펙션 시약 및 시스템 개발자 : 대형 제약회사 구상
제12장 특허 분석
- 챕터 개요
- 범위와 조사 방법
- 비바이러스성 트랜스펙션 시약 및 시스템 : 특허 분석
- 비바이러스성 트랜스펙션 시약 및 시스템 : 특허 벤치마킹 분석
- 비바이러스성 트랜스펙션 시약 및 시스템 : 특허 평가 분석
제13장 출판물 분석
제14장 루트 분석 가격 전략적 프레임워크
제15장 시장 규모 평가와 기회 분석
- 챕터 개요
- 예측 조사 방법과 주요 전제조건
- 비바이러스성 트랜스펙션 시약 및 시스템 시장(-2035년)
- 비바이러스성 트랜스펙션 시약 및 시스템 시장 : 비바이러스성 트랜스펙션법 유형별 분석(-2035년)
- 비바이러스성 트랜스펙션 시약 및 시스템 시장 : 최종사용자별 분석(-2035년)
- 비바이러스성 트랜스펙션 시약 및 시스템 시장 : 용도별 분석(-2035년)
- 비바이러스성 트랜스펙션 시약 및 시스템 시장 : 주요 지역별 분석(-2035년)
제16장 이그제큐티브 인사이트
제17장 결론
제18장 부록 I : 표형식 데이터
제19장 부록 II : 기업 및 조직 리스트
KSA 25.07.10NON-VIRAL TRANSFECTION REAGENTS MARKET: OVERVIEW
As per Roots Analysis, the global non-viral transfection reagents market valued at USD 633 million in the current year is projected to grow at a CAGR of 8.0% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Non-Viral Transfection Method
- Chemical Methods
- Physical Methods
- Other Methods
Application Areas
- Clinical Applications
- Research Applications
End-User
- Academic and Research Institutions
- Pharmaceutical Companies
- Other End-Users
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
NON-VIRAL TRANSFECTION REAGENTS MARKET: GROWTH AND TRENDS
Over the years, a large number of nucleic acid-based therapies have gained regulatory approval, underscoring both their therapeutic potential and rising popularity. As a result, there has been an increased demand for vectors capable of efficiently delivering these therapies. Notably, transfection is one such process of artificially introducing genetic material / nucleic acid into the cells with the use of specific carriers / vectors. However, conventional transfection through viral vectors poses several challenges, such as complications related to immunogenicity and cytotoxicity along with being a cost-intensive development process. Consequently, the demand for alternative gene drug vehicles such as non-viral transfection reagents has increased. The non-viral transfection reagents can overcome limitations, such as inflammation and non-specific transduction, associated with viral vectors. Further, these vectors are significantly cost effective than their virus-based counterparts, implying the potential for use in the development of more affordable products in the gene therapy market.
In non-viral transfection method, the gene transfer to cells may involve either direct introduction of DNA into the host cell, or the delivery of carrier gene by a complex formation with lipids, polymers or lipo-polymers. In both cases, gene sequences are inserted into a plasmid (vector) for its expression within the host. Due to low immunogenicity, non-viral transfection systems allow re-dosing of genetic material without the risk of complications. Moreover, such reagents are more economical than viral vectors as they can be easily produced in large quantities.
NON-VIRAL TRANSFECTION REAGENTS MARKET: KEY INSIGHTS
The report delves into the current state of the non-viral transfection reagents market and identifies potential growth opportunities within industry. Some key findings from the report include:
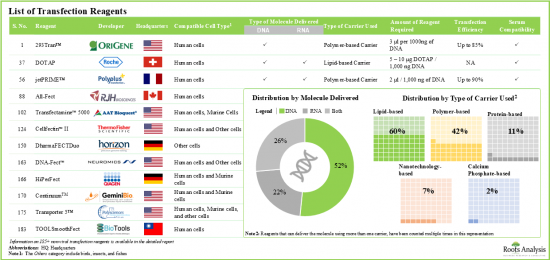
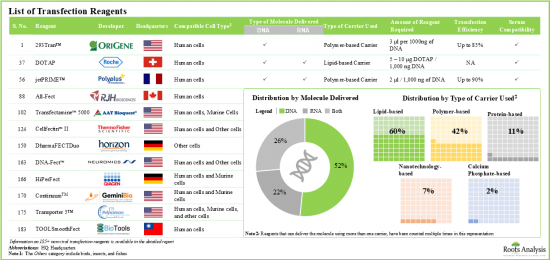
- Over 185 non-viral transfection reagents are currently available in the market; of these, ~60% use lipid-based carriers to enable the transfection of genetic material.
- In pursuit of building a competitive edge, industry stakeholders are actively enhancing their respective offerings to comply with the evolving industry benchmarks.
- Over 20 electroporation-based systems and more than 35 other non-viral transfection systems have been developed for the delivery of DNA, RNA and protein fragments, in different types of cells.
- Developers are focused on the integration of advanced features in their respective systems, in order to maintain their competitive position in the market.
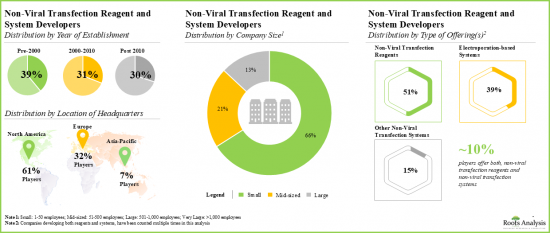
- The current market landscape of non-viral transfection and reagent developers features the presence of both established and emerging players, located across key global regions.
- A notable increase in published scientific literature related to non-viral transfection methods and techniques has been observed in the past few years.
- In the past four years, around 870 patents have been filed / granted related to non-viral transfection methods; of these, over 60% of the patents were by non-academic players.
- Foreseeing a lucrative potential, big pharma players have also undertaken several initiatives focused on non-viral transfection; notably, all collaborations inked by such players in the past two years were focused on preclinical operations.
- In order to optimize the price of advanced therapies, innovators are anticipated to forge alliances with non-viral transfection reagent and system developers.
- Cost is a key determinant for the adoption of non-viral transfection techniques; the pricing strategy framework is likely to assist players in evaluating competitive prices for their reagents.
- The market is likely to witness an annual growth of 8.0%; the projected opportunity is expected to be well distributed across different transfection methods, application areas, end-users and key geographical regions
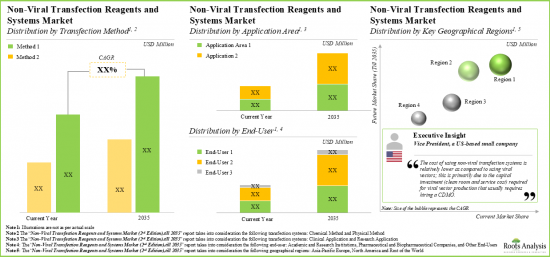
NON-VIRAL TRANSFECTION REAGENTS MARKET: KEY SEGMENTS
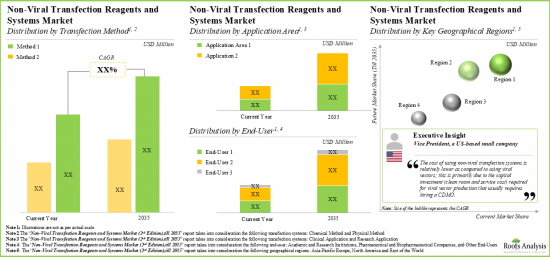
Physical Methods Segment holds the Largest Share of the Non-Viral Transfection Reagents Market
Based on the type of non-viral transfection method, the market is segmented into chemical methods, physical methods and other methods. At present, the physical methods segment holds the maximum share of the global non-viral transfection reagents market. Further, the non-viral transfection reagents market for other methods segment is expected to show the highest market growth potential during the forecast period.
By Application Areas, Clinical Applications is the Fastest Growing Segment of the Global Non-Viral Transfection Reagents Market
Based on the application areas, the market is segmented into clinical applications and research applications. At present, the research applications segment holds the maximum share of the global non-viral transfection reagents market. Further, the market for clinical applications segment is expected to grow at a higher CAGR during the forecast period.
By End-User, Academic and Research Institutions Segment Accounts for the Largest Share of the Global Non-Viral Transfection Reagents Market
Based on the end-user, the market is segmented into academic and research institutions, pharmaceutical companies and other end-users. Currently, the academic and research institutions segment captures the highest proportion of the global non-viral transfection reagents market. However, the pharmaceutical companies segment is expected to grow at a higher CAGR during the forecast period.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific and Rest of the World. Currently, North America dominates the global non-viral transfection reagents market and accounts for the largest revenue share. Further, the market in Asia-Pacific is likely to grow at a higher CAGR in the coming future.
Example Players in the Non-Viral Transfection Reagents Market
- Altogen Biosystems
- Bio-Rad Laboratories
- BEX
- BTX
- Celsion
- Genprex
- Inovio Pharmaceuticals
- MaxCyte
- MilliporeSigma
- Nepa Gene
- OZ Biosciences
- Thermo Fisher Scientific
NON-VIRAL TRANSFECTION REAGENTS MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global non-viral transfection reagents market, focusing on key market segments, including [A] type of non-viral transfection method, [B] application areas, [C] end-user and [D] key geographical regions.
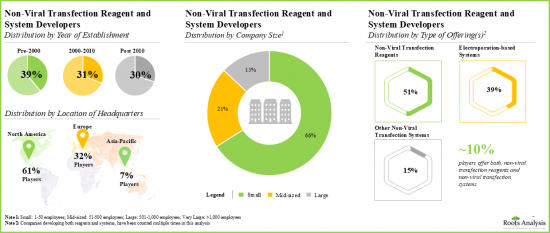
- Non-Viral Transfection Reagents Market Landscape: A comprehensive evaluation of the companies developing non-viral transfection reagents, based on several relevant parameters, such as [A] type of carrier used, [B] compatible cell type, [C] type of molecule delivered and [D] serum compatibility. Additionally, a comprehensive evaluation of non-viral transfection reagent developers, based on several relevant parameters, such as [A] year of establishment, [B] company size and [C] location of headquarters.
- Electroporation-Based Transfection Systems Market Landscape: A detailed analysis of the companies developing electroporation-based non-viral transfection systems, based on several relevant parameters, such as [A] compatible cell type and [B] type of molecule delivered. Additionally, a comprehensive evaluation of electroporation transfection systems developers, based on several relevant parameters, such as [A] year of establishment, [B] company size and [C] location of headquarters.
- Other Non-Viral Transfection Systems Market Landscape: An insightful evaluation of the companies developing other non-viral transfection systems, based on several relevant parameters, such as [A] compatible cell type and [B] type of molecule delivered. Additionally, a comprehensive evaluation of other non-viral transfection systems developers, based on several relevant parameters, such as [A] year of establishment, [B] company size and [C] location of headquarters.
- Company Competitiveness Analysis: A comprehensive competitive analysis of non-viral transfection reagent and system developers, examining factors, such as [A] developer strength and [B] product portfolio strength.
- Technology Competitiveness Analysis: A detailed analysis of electroporation transfection systems and other non-viral transfection systems, examining factors, such as [A] developer strength and [B] product portfolio strength.
- Company Profiles: In-depth profiles of key players that are engaged in the development of non-viral transfection reagents and systems, focusing on [A] overview of the company, [B] financial information (if available) and [C] recent developments and an informed future outlook.
- Potential Strategic Partners: An insightful analysis of more than 80 cell and gene therapy developers that are likely to partner with non-viral transfection reagent and system developers, based on several relevant parameters, such as [A] pipeline maturity, [B] developer strength, [C] pipeline strength and [D] type of therapy.
- Benchmark Analysis: A comprehensive benchmark analysis of various non-viral focused initiatives undertaken by big pharma players, based on several relevant parameters, such as [A] year of initiative, [B] type of initiative, [C] type of therapy and [D] target therapeutic area.
- Patent Analysis: An in-depth analysis of patents filed / granted till date in the non-viral transfection systems domain, based on various relevant parameters, such as [A] type of patent, [B] publication year, [C] application year, [D] geography, [E] type of applicant, [F] CPC symbols, [G] emerging focus areas, [H] leading players (in terms of number of patents granted / filed), [I] patent benchmarking and [J] valuation analysis.
- Publication Analysis: An insightful analysis of over 450 peer-reviewed, scientific articles focused on non-viral transfection reagents and systems, based on various relevant parameters, such as [A] year of publication, [B] type of publication, [C] type of molecule delivered, [D] target therapeutic area, [E] key focus areas, [F] popular cells and cell lines. Additionally, the section includes analysis of the leading publishers across different geographies and key journals (in terms of number of articles published).
- Roots Analysis Pricing Strategy Framework: A detailed framework for analyzing the pricing strategy of a company's non-viral transfection reagents and its competitive market position, including an equation to estimate reagent prices based on their characteristics.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Introduction to Transfection
- 3.3. Methods of Transfection
- 3.3.1. Viral Transfection Systems
- 3.3.1.1. Types of Viral Transfection Vectors
- 3.3.1.1.1. Adeno-associated Virus-based Vectors
- 3.3.1.1.2. Adenovirus-based Vectors
- 3.3.1.1.3. Herpes Virus-based Vectors
- 3.3.1.1.4. Lentivirus-based Vectors
- 3.3.1.1.5. Retroviral-based Vectors
- 3.3.1.1. Types of Viral Transfection Vectors
- 3.3.2. Non-Viral Transfection Systems
- 3.3.2.1. Chemical-based Transfection
- 3.3.2.1.1. Lipoplexes-based Transfection
- 3.3.2.1.2. Polyplexes-based Transfection
- 3.3.2.1.3. Lipo-polyplexes-based Transfection
- 3.3.2.1.4. Dendrimer-based Transfection
- 3.3.2.1.5. Cell Penetrating Peptide-based Transfection
- 3.3.2.2. Physical Transfection Systems
- 3.3.2.2.1. Electroporation-based Transfection
- 3.3.2.2.2. Gene Gun-based Transfection
- 3.3.2.2.3. Sonoporation-based Transfection
- 3.3.2.2.4. Microinjection-based Transfection
- 3.3.2.2.5. Magnetofection-based Transfection
- 3.3.2.1. Chemical-based Transfection
- 3.3.1. Viral Transfection Systems
- 3.4. Applications of Transfection
- 3.4.1. Advanced Therapeutic Medicinal Product Development
- 3.4.2. Gene Silencing
- 3.4.3. Generation of Stable Cell Lines
- 3.4.4. Large-scale Protein Production
- 3.4.5. Stem Cell Engineering
- 3.5. Future Perspectives
4. NON-VIRAL TRANSFECTION REAGENTS: MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. List of Non-Viral Transfection Reagents
- 4.2.1. Analysis by Type of Carrier Used
- 4.2.2. Analysis by Compatible Cell Type
- 4.2.3. Analysis by Type of Molecule Delivered
- 4.2.4. Analysis by Serum Compatibility
- 4.3 List of Non-Viral Transfection Reagent Developers
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Location of Headquarters (Region-wise)
- 4.3.4. Analysis by Location of Headquarters (Country-wise)
5. ELECTROPORATION-BASED TRANSFECTION SYSTEMS: MARKET LANDSCAPE
- 5.1. Chapter Overview
- 5.2. List of Electroporation-based Transfection Systems
- 5.2.1. Analysis by Compatible Cell Type
- 5.2.2. Analysis by Type of Molecule Delivered
- 5.3. List of Electroporation-based Transfection System Developers
- 5.3.1. Analysis by Year of Establishment
- 5.3.2. Analysis by Company Size
- 5.3.3. Analysis by Location of Headquarters (Region -wise)
- 5.3.4. Analysis by Location of Headquarters (Country-wise)
6. OTHER NON-VIRAL TRANSFECTION SYSTEMS: MARKET LANDSCAPE
- 6.1. Chapter Overview
- 6.2. List of Other Non-Viral Transfection Systems
- 6.2.1. Analysis by Compatible Cell Type
- 6.2.2. Analysis by Type of Molecule Delivered
- 6.3. List of Other Non-Viral Transfection System Developers
- 6.3.1. Analysis by Year of Establishment
- 6.3.2. Analysis by Company Size
- 6.3.3. Analysis by Location of Headquarters (Region-wise)
- 6.3.4. Analysis by Location of Headquarters (Country-wise)
7. COMPANY COMPETITIVENESS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Methodology and Key Parameters
- 7.3. Non-Viral Transfection Reagent Developers: Company Competitiveness Analysis
- 7.3.1. Non-Viral Transfection Reagent Developers based in North America
- 7.3.2. Non-Viral Transfection Reagent Developers based in Europe
- 7.3.3. Non-Viral Transfection Reagent Developers based in Asia-Pacific and Rest of the World
8. TECHNOLOGY COMPETITIVENESS ANALYSIS
- 8.1. Chapter Overview
- 8.2. Methodology and Key Parameters
- 8.3. Electroporation-based Transfection Systems: Technology Competitiveness Analysis
- 8.3.1. Technologies Offered by Players based in North America
- 8.3.2. Technologies Offered by Players based in Europe
- 8.3.3. Technologies Offered by Players based in Asia-Pacific and Rest of the World
- 8.4. Other Non-Viral Transfection Systems: Technology Competitiveness Analysis
- 8.4.1. Technologies Offered by Players based in North America
- 8.4.2. Technologies Offered by Players based in Europe
- 8.4.3. Technologies Offered by Players based in Asia-Pacific and Rest of the World
9. COMPANY PROFILES
- 9.1. Chapter Overview
- 9.2. Non-Viral Transfection Reagent Developers
- 9.2.1. MilliporeSigma
- 9.2.1.1. Company Overview
- 9.2.1.2. Financial Information
- 9.2.1.3. Recent Developments and Future Outlook
- 9.2.2. OZ Biosciences
- 9.2.2.1. Company Overview
- 9.2.2.2. Recent Developments and Future Outlook
- 9.2.3. Thermo Fisher Scientific
- 9.2.3.1. Company Overview
- 9.2.3.2. Financial Information
- 9.2.3.3. Recent Development and Future Outlook
- 9.2.1. MilliporeSigma
- 9.3. Electroporation-based Transfection System Developers
- 9.3.1. BEX
- 9.3.1.1. Company Overview
- 9.3.1.2. Recent Developments and Future Outlook
- 9.3.2. Bio-Rad Laboratories
- 9.3.2.1. Company Overview
- 9.3.2.2. Financial Information
- 9.3.2.3. Recent Developments and Future Outlook
- 9.3.3. BTX (A subsidiary of Harvard Bioscience)
- 9.3.3.1. Company Overview
- 9.3.3.2. Recent Developments and Future Outlook
- 9.3.4. MaxCyte
- 9.3.4.1. Company Overview
- 9.3.4.2. Financial Information
- 9.3.4.3. Recent Developments and Future Outlook
- 9.3.5. NepaGene
- 9.3.5.1. Company Overview
- 9.3.5.2. Recent Developments and Future Outlook
- 9.3.1. BEX
- 9.4. Other Non-Viral Transfection System Developers
- 9.4.1. Imunon (Formerly known as Celsion)
- 9.4.1.1. Company Overview
- 9.4.1.2. Recent Developments and Future Outlook
- 9.4.2. Genprex
- 9.4.2.1. Company Overview
- 9.4.2.2. Financial Information
- 9.4.2.3. Recent Developments and Future Outlook
- 9.4.3. Inovio Pharmaceuticals
- 9.4.3.1. Company Overview
- 9.4.3.2. Financial Information
- 9.4.3.3. Recent Developments and Future Outlook
- 9.4.1. Imunon (Formerly known as Celsion)
10. POTENTIAL STRATEGIC PARTNERS
- 10.1. Chapter Overview
- 10.2. Scope and Methodology
- 10.3. Non-Viral Transfection System Developers: Potential Strategic Partners in North America
- 10.3.1. Most Likely Partners
- 10.3.2. Likely Partners
- 10.3.3. Less Likely Partners
- 10.3.4. Least Likely Partners
- 10.4. Non-Viral Transfection System Developers: Potential Strategic Partners in Europe
- 10.4.1. Most Likely Partners
- 10.4.2. Likely Partners
- 10.4.3. Less Likely Partners
- 10.5. Non-Viral Transfection System Developers: Potential Strategic Partners in Asia-Pacific and Rest of the World
- 10.5.1. Most Likely Partners
- 10.5.2. Likely Partners
- 10.5.3. Less Likely Partners
11. BIG PHARMA INITIATIVES
- 11.1. Chapter Overview
- 11.2. Scope and Methodology
- 11.3. None-Viral Transfection Reagents and System Developers: Big Pharma Initiatives
- 11.3.1. Analysis by Year of Initiative
- 11.3.2. Analysis by Number of Initiative
- 11.3.3. Analysis by Type of Initiative
- 11.3.4. Analysis by Type of Therapy
- 11.3.5. Analysis by Target Therapeutic Area
12. PATENT ANALYSIS
- 12.1. Chapter Overview
- 12.2. Scope and Methodology
- 12.3. Non-Viral Transfection Reagents and Systems: Patent Analysis
- 12.3.1. Analysis by Publication Year
- 12.3.2. Analysis by Application Year
- 12.3.3. Analysis by Patent Jurisdiction
- 12.3.4. Analysis by Type of Applicant
- 12.3.5. Analysis by CPC Sections
- 12.3.6. Analysis by Emerging Focus Areas (Word Cloud Representation)
- 12.3.7. Leading Players: Analysis by Number of Patents
- 12.4. Non-Viral Transfection Reagents and Systems: Patent Benchmarking Analysis
- 12.4.1. Analysis by Patent Characteristics (CPC Symbols)
- 12.4.2. Analysis by Geography
- 12.5. Non-Viral Transfection Reagents and Systems: Patent Valuation Analysis
13. PUBLICATION ANALYSIS
- 13.1. Chapter Overview
- 13.2. Scope and Methodology
- 13.3. Non-Viral Transfection Reagents and Systems: Recent Publications
- 13.4. Analysis by Year of Publication
- 13.5. Analysis by Type of Publication
- 13.6. Analysis by Type of Molecule Delivered
- 13.7. Analysis by Target Therapeutic Area
- 13.8. Analysis by Key Focus Areas (Word Cloud Representation)
- 13.9. Analysis by Prominent Cells and Cell Lines (Word Cloud Representation)
- 13.10. Leading Publishers: Analysis by Number of Publications
- 13.11. Prominent Journals: Analysis by Number of Publications
- 13.12. Prominent Copyright Holders: Analysis by Number of Publications
- 13.13. Key Funding Institutes: Analysis by Number of Publications
14. ROOTS ANALYSIS PRICING STRATEGY FRAMEWORK
- 14.1. Chapter Overview
- 14.2. Roots Analysis Framework
- 14.2.1. Methodology
- 14.2.2. Theoretical Framework and Price Evaluation Hypothesis
- 14.2.3. Results and Interpretation
- 14.2.3.1. Product Price Evaluation Matrix: Based on Transfection Efficiency
- 14.2.3.2. Product Price Evaluation Matrix: Based on Compatible Cell Type
- 14.2.3.3. Product Price Evaluation Matrix: Based on Type of Carrier Used
- 14.2.3.4. Product Price Evaluation Matrix: Based on Type of Molecule Delivered
- 14.2.3.5. Product Price Evaluation Matrix: Based on Serum Compatibility
- 14.3. Concluding Remarks
15. MARKET SIZING AND OPPORTUNITY ANALYSIS
- 15.1. Chapter Overview
- 15.2. Forecast Methodology and Key Assumptions
- 15.3. Non-Viral Transfection Reagents and Systems Market, Till 2035
- 15.4. Non-Viral Transfection Reagents and Systems Market: Analysis by Type of Non-Viral Transfection Method, Till 2035
- 15.5. Non-Viral Transfection Reagents and Systems Market: Analysis by End-User, Till 2035
- 15.6. Non-Viral Transfection Reagents and Systems Market: Analysis by Application Area, Till 2035
- 15.7. Non-Viral Transfection Reagents and Systems Market: Analysis by Key Geographical Regions, Till 2035
- 15.7.1. Non-Viral Transfection Reagents and Systems Market in North America, Till 2035
- 15.7.1.1. Non-Viral Transfection Reagents and Systems Market in North America: Analysis by Type of End-User, Till 2035
- 15.7.1.1.1. Non-Viral Transfection Reagents and Systems Market in North America for Pharmaceutical Companies, Till 2035
- 15.7.1.1.2. Non-Viral Transfection Reagents and Systems Market in North America for Academic and Research Institutions, Till 2035
- 15.7.1.1.3. Non-Viral Transfection Reagents and Systems Market in North America for Other End-Users, Till 2035
- 15.7.1.2. Non-Viral Transfection Reagents and Systems Market in North America: Analysis by Application Area, Till 2035
- 15.7.1.2.1. Non-Viral Transfection Reagents and Systems Market in North America for Research Applications, Till 2035
- 15.7.1.2.2. Non-Viral Transfection Reagents and Systems Market in North America for Clinical Applications, Till 2035
- 15.7.1.1. Non-Viral Transfection Reagents and Systems Market in North America: Analysis by Type of End-User, Till 2035
- 15.7.2. Non-Viral Transfection Reagents and Systems Market in Europe, Till 2035
- 15.7.2.1. Non-Viral Transfection Reagents and Systems Market in Europe: Analysis by Type of End-User, Till 2035
- 15.7.2.1.1. Non-Viral Transfection Reagents and Systems Market in Europe for Pharmaceutical Companies, Till 2035
- 15.7.2.1.2. Non-Viral Transfection Reagents and Systems Market in Europe for Academic and Research Institutions, Till 2035
- 15.7.2.1.3. Non-Viral Transfection Reagents and Systems Market in Europe for Other End-Users, Till 2035
- 15.7.2.2. Non-Viral Transfection Reagents and Systems Market in Europe: Analysis by Application Area, Till 2035
- 15.7.2.2.1. Non-Viral Transfection Reagents and Systems Market in Europe for Research Applications, Till 2035
- 15.7.2.2.2. Non-Viral Transfection Reagents and Systems Market in Europe for Clinical Applications, Till 2035
- 15.7.2.1. Non-Viral Transfection Reagents and Systems Market in Europe: Analysis by Type of End-User, Till 2035
- 15.7.3. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific, Till 2035
- 15.7.3.1. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific: Analysis by Type of End-User, Till 2035
- 15.7.3.1.1. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific for Pharmaceutical Companies, Till 2035
- 15.7.3.1.2. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific for Academic and Research Institutions, Till 2035
- 15.7.3.1.3. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific for Other End-Users, Till 2035
- 15.7.3.2. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific: Analysis by Application Area, Till 2035
- 15.7.3.2.1. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific for Research Applications, Till 2035
- 15.7.3.2.2. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific for Clinical Applications, Till 2035
- 15.7.3.1. Non-Viral Transfection Reagents and Systems Market in Asia-Pacific: Analysis by Type of End-User, Till 2035
- 15.7.4. Non-Viral Transfection Reagents and Systems Market in Rest of the World, Till 2035
- 15.7.4.1. Non-Viral Transfection Reagents and Systems Market in Rest of the World: Analysis by Type of End-User, Till 2035
- 15.7.4.1.1. Non-Viral Transfection Reagents and Systems Market in Rest of the World for Pharmaceutical Companies, Till 2035
- 15.7.4.1.2. Non-Viral Transfection Reagents and Systems Market in Rest of the World for Academic and Research Institutions, Till 2035
- 15.7.4.1.3. Non-Viral Transfection Reagents and Systems Market in Rest of the World for Other End-Users, Till 2035
- 15.7.4.2. Non-Viral Transfection Reagents and Systems Market in Rest of the World: Analysis by Application Area, Till 2035
- 15.7.4.2.1. Non-Viral Transfection Reagents and Systems Market in Rest of the World for Research Applications, Till 2035
- 15.7.4.2.2. Non-Viral Transfection Reagents and Systems Market in Rest of the World for Clinical Applications, Till 2035
- 15.7.4.1. Non-Viral Transfection Reagents and Systems Market in Rest of the World: Analysis by Type of End-User, Till 2035
- 15.7.1. Non-Viral Transfection Reagents and Systems Market in North America, Till 2035



















