
|
시장보고서
상품코드
1771292
Fc 융합 단백질 시장 : 업계 동향과 세계 예측 - 표적 적응증별, 융합 분자 유형별, 투여 경로별, 주요 지역별Fc Fusion Protein Market: Industry Trends and Global Forecasts - Distribution by Target Indications, Type of Fusion Molecule, Route of Administration and Key Geographical Regions |
||||||
세계의 Fc 융합 단백질 시장 : 개요
세계 Fc 융합 단백질 시장 규모는 올해 155억 달러에 달했습니다. 이 시장은 예측 기간 동안 10%의 연평균 복합 성장률(CAGR)로 확대될 것으로 예측됩니다.
시장 세분화 및 기회 분석은 다음과 같은 매개 변수로 세분화됩니다.
표적 적응증
- 호중구 감소증
- 이식편대 숙주병
- 유방암
- 류마티스 관절염
- 비소세포폐암
- 신생혈관성(습성) 노인성 황반변성(AMD)
- 혈우병 A
- 시신경 척수염 스펙트럼 장애
- 전신성 홍반성 루푸스
융합 분자 유형
- 항체
- 사이토카인
- 성장인자
- 리셉터클 ECD
- 기타
투여 경로
- 피하
- 정맥 투여
- 유리체 내
주요 지역
- 북미
- 유럽
- 아시아태평양
- 기타 지역
세계의 Fc 융합 단백질 시장 : 성장과 트렌드
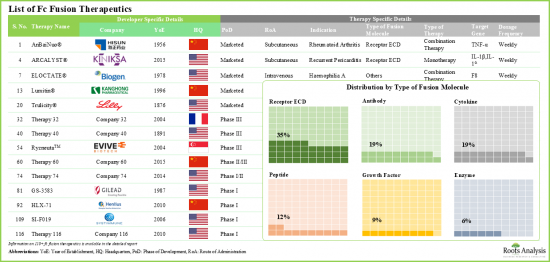
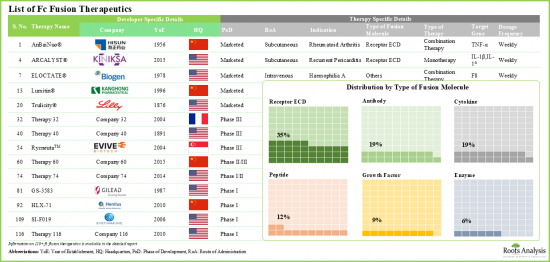
1998년 류마티스 관절염 치료제 Enbrel(R)(CD4-Fc 융합 단백질)이 승인된 이후, Fc 융합 단백질 치료는 대표적인 치료제로 자리 잡았습니다. 현재 Fc 융합 단백질을 기반으로 한 13종의 약물이 시판되고 있으며, 약 50여종의 분자가 다양한 질환 적응증을 위해 개발 중에 있습니다. 이러한 치료제는 생물학적 활성 리간드의 유리한 약리학적 특성과 면역글로불린 G(IgG)의 결정화 가능한 단편(Fc) 도메인의 고유한 특성을 결합한 것입니다. 또한, 생물학적 활성 단백질의 혈청 내 반감기를 연장하는 능력으로 인해 이러한 질병 변형 치료제는 다양한 치료 분야에서 활용되고 있습니다. 여기에는 종양, 신경, 호흡기, 희귀 유전성 질환 등이 포함됩니다.
현재 수많은 의약품 개발자들이 효능을 향상시킨 새로운 Fc 융합 치료제의 개발에 적극적으로 나서고 있습니다. 실제로 이 분야 개발자들의 연구 노력의 핵심은 약리학적으로 활성 성분의 안정성과 용해도를 향상시켜 궁극적으로 치료 가능성을 높이는 데 있습니다. 그 결과, Fc 융합치료제 시장은 예측 기간 동안 건강한 시장 성장을 보일 것으로 예측됩니다.
세계의 Fc 융합 단백질 시장 : 주요 인사이트
이 보고서는 세계 Fc 융합 단백질 시장의 현황을 조사하고 업계 내 잠재적인 성장 기회를 파악합니다. 본 보고서의 주요 조사 결과는 다음과 같습니다.
- 현재 전 세계 약 40개 기업이 다양한 적응증에 대한 Fc 융합 치료제의 잠재적 이점을 평가하기 위해 노력하고 있습니다.
- 파이프라인에는 115개 이상의 약물이 개발 단계에 따라 단독으로 또는 다른 치료제와 병용하여 평가되고 있으며, 대부분 비경구 투여용으로 설계되어 있습니다.
- 승인된 치료제 및 후기 단계 후보물질의 대부분은 다양한 종양질환, 유전질환, 혈액질환, 면역질환의 치료를 목적으로 하고 있습니다.
- fc 융합의 치료적 이점을 고려할 때, 이러한 중재는 주로 단일 요법으로 평가되고 있습니다. 단독요법으로 연구되고 있는 후기 단계의 약물의 예로는 ACE-011과 RC18이 있습니다.
- fc 융합 치료제의 약 50%는 피하 투여용이며, 다양한 약물 전달 시스템을 통해 환자가 자가 투여할 수 있습니다.
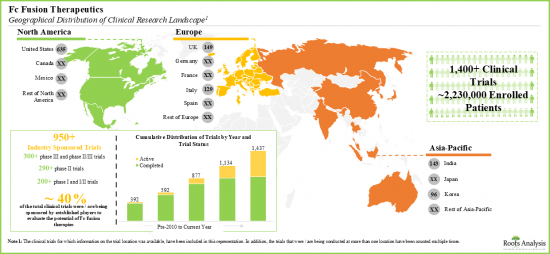
- 다양한 지역에서 120개에 가까운 fc 융합 기반 치료제 후보물질의 평가를 위해 실시되고 있는 임상시험에 2백만 명 이상의 환자가 등록되어 있습니다.
- 이 분야의 연구 노력을 지원하기 위해 여러 기관에서 재정적 지원을 하고 있습니다. 현재 지출된 자금은 주로 SBIR/STTR 이외의 목적의 약물 연구 지원에 중점을 두고 있습니다.
- 이 분야의 이해관계자들에게 수여되는 보조금의 수는 최근 몇 년 동안 증가하고 있으며, 총액의 60% 이상이 연구 프로젝트에 수여되고 있습니다.
- 이 분야에는 NIH의 다양한 관리기관이 참여하고 있는데, 그 중에서도 NCI, NIAID, NHLBI의 참여가 상대적으로 두드러집니다.
- 시간이 지남에 따라 Fc 융합 치료제 관련 지적재산권은 산업계와 비산업계 모두에서 특허를 출원하여 칭찬할 만한 속도로 성장하고 있습니다.
- 이 분야의 특허 출원/취득 건수는 해당 기간 동안 CAGR 17%로 증가했으며, 대부분 지난 2년간 출원/취득된 특허가 대부분을 차지했습니다.
- 업계 관계자 외에도 스탠포드대, INSERM 등 학계에서도 fc 융합치료제 관련 특허를 출원하고 있습니다.
- 가치가 높은 특허는 수용체 ECD, 효소, 펩타이드 등 다양한 융합 분자별 Fc 영역의 변형에 초점을 맞추었습니다.
- 발표된 과학 문헌은 이 분야의 연구 진행 속도를 보여줍니다. 과거와 현재 진행 중인 연구의 초점은 항암제 개발에 집중되어 있는 것으로 보입니다.
- 지난 몇 년 동안 fc 융합 치료제 관련 논문이 꾸준히 증가하고 있습니다. 여기에는 항체의 Fc 영역에 융합된 생물학적 부위의 유형에 초점을 맞춘 논문도 포함되어 있습니다.
- 발표된 논문의 대부분(-70%)은 다양한 암 및 혈액 질환에서 Fc 융합 단백질의 치료 가능성을 평가하는 데 초점을 맞춘 조사 연구에 관한 것입니다.
- Fc 융합치료제 관련 논문은 영향력 있는 여러 저널에 게재되고 있지만, PLoS One과 MAbs가 30편 이상의 논문을 게재하는 주요 저널로 부상하고 있습니다.
- 개발 중기 및 후기 단계에 있는 여러 파이프라인 후보물질은 주로 종양성 질환을 대상으로 하며, 대부분 Fc 단백질을 조작한 항체입니다.
- Fasenra, Gazyva, Margenza, Skyrizi와 같은 많은 시판된 약제들은 현재 다른 종양학적 적응증에서도 그 효과를 평가받고 있습니다.
- 후기 단계 후보물질의 대부분(-60%)은 고형암, 비호지킨림프종, 비소세포폐암을 포함한 종양학적 적응증을 대상으로 하고 있습니다.
- 파이프라인에 있는 후기 단계 치료제 증가에 힘입어 수익 측면에서 미래의 기회는 연간 10%에 가까운 성장률을 보일 것으로 예측됩니다.
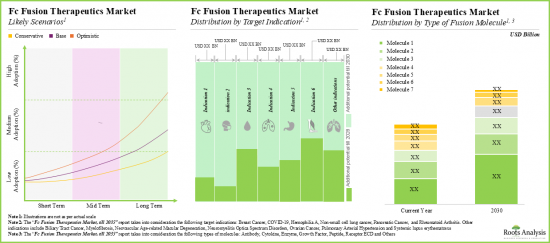
- 추정된 시장 기회는 다양한 유형의 치료법, 표적 적응증, 융합 분자 유형, 투여 경로, 주요 지역별로 고르게 분포되어 있을 것으로 예측됩니다.
신약개발 플랫폼 시장 진출기업 사례
- Abzena
- Creative Biolabs
- Distributed Bio (Subsidiary of Charles River)
- ImmunoPrecise Antibodies
- IONTAS
- LakePharma
- Leading Biology
- Sino Biological
- XOMA
세계의 Fc 융합 단백질 시장
- 시장 규모 및 기회 분석 : 이 보고서는 세계 Fc 융합 단백질 시장을 상세하게 분석하고,(A) 표적 적응증,(B) 융합 분자 유형,(C) 투여 경로,(D) 주요 지역 등 주요 시장 부문에 초점을 맞추었습니다.
- 시장 상황: A) 리드 후보물질의 개발 단계, B) 융합 분자 유형, C) 표적 유전자, D) 치료 영역, E) 표적 질환의 적응증, F) 치료법 유형, G) 투여 경로, H) 투여 횟수 등 다양한 파라미터를 고려한 Fc 융합 치료제를 종합적으로 평가합니다. 또한,(A) 설립연도,(B) 기업 규모,(C) 본사 소재지를 기준으로 의약품 개발 기업을 세부적으로 평가합니다.
- 기업 프로파일:(A) 기업 개요,(B) 재무 정보(가능한 경우),(C) 제품 포트폴리오,(D) 최근 동향 및 정보에 기반한 미래 전망에 초점을 맞춘 Fc 융합 치료제 개발에 종사하는 기업의 상세한 프로파일.
- 임상시험 분석 :(A)시험 등록 연도,(B)시험 단계,(C)시험 설계,(D)마스킹 유형,(E)중재 모델 유형,(F)새로운 중점 분야,(G)주요 산업 스폰서/공동 연구자,(H)인기 있는 적응증,(i)인기 있는 중재,(J)시험의 지역적 분포 등. 몇 가지 관련 매개 변수를 기반으로 다양한 Fc 융합 치료제의 임상시험에 대한 자세한 분석.
- 보조금 분석 : A) 보조금 수여 연도, B) 보조금 수여 금액, C) 관리 기관 센터, D) 지원 기간, E) 보조금 신청 유형, F) 보조금 수여 목적, G) 활동 코드, H) 새로운 중점 분야 등 다양한 관련 매개 변수를 기반으로 Fc 융합치료제 관련 프로젝트를 수행하는 연구 기관에 수여된 보조금에 대한 상세 분석.
- 출판물 분석 : A)발표 연도,(B)새로운 중점 분야,(C)표적 치료 분야,(D)주요 저자,(E)주요 저널을 기준으로 Fc 융합 치료제와 관련된 약 1,135건의 피어 리뷰 과학 논문을 종합적으로 분석.
- 특허 분석 :(A) 공개연도,(B) 지역,(C) CPC 기호,(D) 새로운 중점 분야,(E) 출원인 유형,(F) 주요 산업 진출기업,(G) 특허 평가 분석 등 다양한 관련 파라미터를 기반으로 Fc 융합치료제 관련 출원/허가된 특허를 상세하게 분석합니다.
- 파트너십 및 협업 A) 파트너십 체결 연도,(B) 파트너십 유형,(C) 중점 분야,(D) 파트너 유형,(E) 가장 활발한 진출 기업(파트너십 체결 건수),(F) 파트너십 활동의 지역별 분포 등 몇 가지 매개 변수를 기반으로 이 분야의 이해관계자들이 체결한 거래에 대한 통찰력 있는 분석.
- 사례 연구 A) 개발 단계,(B) 대상 질환,(C) 치료 영역,(D) Fc 엔지니어링 유형,(E) Fc 엔지니어링의 영향,(F) 투여 경로,(G) 치료 유형 등 여러 관련 매개 변수를 기반으로 시판 또는 개발 중인 Fc 단백질 엔지니어링 및 당 사슬 엔지니어링 및 당쇄 엔지니어링에 대한 상세한 고찰.
세계의 Fc 융합 단백질 시장에 대해 조사했으며, 시장 개요와 함께 표적 적응증별/융합 분자 유형별/투여 경로별 동향, 지역별 동향, 시장 진출기업 프로파일 등의 정보를 전해드립니다.
목차
제1장 서문
제2장 주요 요약
제3장 서론
- Fc 융합 치료제 개요
- Fc 융합 치료제 성분
- 작용기전
- Fc 융합 치료제 유형
- Fc 융합 치료제 응용
- Fc 융합 치료제가 기타 생물학적 분자에 대해서 가지는 이점
- 향후 전망
제4장 파이프라인 리뷰 : 시판약과 임상약
- 분석 조사 방법과 주요 파라미터
- Fc 융합 치료제 : 의약품 파이프라인
- Fc 융합 치료제 : 파이프라인 분석
- Fc 융합 치료제 : 의약품 개발 기업 리스트
제5장 기업 개요
- 본 장의 개요
- Alphamab Oncology
- Amgen
- Acceleron Pharmaceuticals
- Bristol Myers Squibb
- Sanofi
제6장 임상시험 분석
- 분석 조사 방법과 주요 파라미터
- Fc 융합 치료제 : 임상시험 리스트
제7장 학술 보조금 분석
- 분석 조사 방법과 주요 파라미터
- Fc 융합 치료제 : 학술 보조금 분석
제8장 출판물 분석
제9장 특허 분석
- 분석 조사 방법과 주요 파라미터
- Fc 융합 치료제 : 특허 분석
제10장 파트너십 및 협업
- 분석 조사 방법과 주요 파라미터
- 파트너십 모델
- Fc 융합 치료제 : 파트너십 및 협업 리스트
제11장 시장 규모 평가와 기회 분석
- 예측 조사 방법과 주요 전제조건
- 세계의 Fc 융합 치료제 시장(2035년까지)
- 세계의 Fc 융합 치료제 시장(-2030년) : 표적 적응증별
- 세계의 Fc 융합 치료제 시장(-2030년) : 융합 분자 유형별
- 세계의 Fc 융합 치료제 시장(-2030년) : 치료 유형별
- 세계의 Fc 융합 치료제 시장(-2030년) : 투여 경로별
- 세계의 Fc 융합 치료제 시장(-2030년) : 지역별
- Fc 융합 치료제 : 개별 제품 판매 예측
- ABP 938 (Amgen)
- Alprolix(R) (Sanofi)
- AnBaiNuo(R) (Hisun Pharmaceuticals)
- Arcalyst(R) (Kiniska Pharmaceuticals)
- BIVV001 (Sanofi)
- CD24Fc (Merck)
- Eloctate(R) (Biogen)
- Eylea(TM) (Regeneron Pharmaceuticals)
- FRSW107 (Zhengzhou Gensciences)
- KN035 (Alphamab Oncology)
- KN046 (Alphamab Oncology)
- Lumitin(R) (Chengdu Kanghong Biotech)
- Reblozyl(R) (Bristol-Myers Squibb)
- RyzneutaTM (Evive Biotech)
- Strensiq(R) (AstraZeneca)
- Telitacicept (RemeGen)
제12장 사례 연구 : FC 단백질 엔지니어링 및 당쇄공학 항체
- Fc 단백질 엔지니어링 및 당쇄공학 항체 : 의약품 파이프라인
- Fc 단백질 엔지니어링 및 당쇄공학 항체 : 개발자 리스트
제13장 결론
제14장 부록 1 : 표 형식 데이터
제15장 부록 2 : 기업 및 단체 리스트
LSH 25.07.22GLOBAL FC FUSION PROTEIN MARKET: OVERVIEW
As per Roots Analysis, the global Fc fusion protein market valued at USD 15.5 billion in the current year is anticipated to grow at a CAGR of 10% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Target Indications
- Neutropenia
- Graft Versus Host Disease
- Breast Cancer
- Rheumatoid Arthritis
- Non-Small Cell Lung Cancer
- Neovascular (Wet) Age-related Macular Degeneration (AMD)
- Hemophilia A
- Neuromyelitis Optica Spectrum Disorders
- Systemic Lupus Erythematosus
Type of Fusion Molecule
- Antibody
- Cytokine
- Growth Factor
- Receptor ECD
- Others
Route of Administration
- Subcutaneous
- Intravenous
- Intravitreal
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
GLOBAL FC FUSION PROTEIN MARKET: GROWTH AND TRENDS
Since the approval of Enbrel(R) (a CD4-Fc fusion protein for the treatment of rheumatoid arthritis) in 1998, Fc fusion protein therapies have become a prominent class of therapeutics. At present, 13 Fc fusion protein-based drugs are commercially available, and approximately 50 additional molecules are in development for a range of disease indications. These therapies combine the advantageous pharmacological properties of biologically active ligands with the unique characteristics of the crystallizable fragment (Fc) domain of immunoglobulin G (IgG). Moreover, due to their ability to prolong the serum half-life of biologically active proteins, these disease-modifying therapies are utilized in a variety of therapeutic areas. Some of these include oncological, neurological, respiratory, and rare genetic disorders.
Currently, numerous drug developers are actively involved in the development of novel Fc fusion therapies with improved efficacy. In fact, the research efforts by developers in this area are centered on enhancing the stability and solubility of the pharmacologically active component, ultimately aiming to boost its therapeutic potential. Consequently, the Fc fusion therapies market is expected to witness healthy market growth during the forecast period.
GLOBAL FC FUSION PROTEIN MARKET: KEY INSIGHTS
The report delves into the current state of global Fc fusion protein market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Presently, around 40 players from across the world, are engaged in evaluating the potential benefits of Fc fusion therapeutics for the treatment of a wide range of disease indications.
- The pipeline features 115+ drug therapies being evaluated either as monotherapies or in combination with other interventions across different stages of development; most of these are designed for parenteral administration.
- Majority of the approved therapies and late-stage candidates are intended for the treatment of various oncological disorders, genetic disorders, hematological disorders and immunological disorders.
- Given the therapeutic benefits of fc fusion, these interventions are primarily evaluated as monotherapies; examples of late-stage drugs being investigated as monotherapy include ACE-011 and RC18.
- Around 50% of the fc fusion therapeutics are meant for subcutaneous administration; these can be self-administered by the patients using different drug delivery systems.
- Over two million patients have been enrolled in the clinical studies being conducted for the evaluation of close to 120 fc fusion-based therapy candidates, across various geographies.
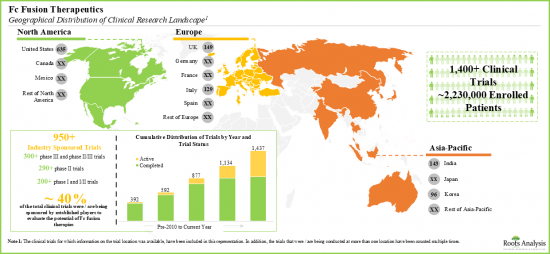
- Several organizations have extended financial support to aid research efforts in this domain; currently, the focus, in terms of funds disbursed, is primarily in support of investigations of drugs for non-SBIR / STTR purposes.
- The number of grants awarded to stakeholders in this domain have increased in the past few years; more than 60% of the total amount was awarded for research projects.
- The field has witnessed the involvement of various administering institutes of the NIH; of all the institutes, participation of the NCI, NIAID, and NHLBI has been relatively more prominent.
- Over time, the intellectual property related to Fc fusion therapeutics has grown at a commendable pace, with patents being filed by both industry and non-industry players.
- Number of patents filed / granted in this domain have increased at a CAGR of 17% during the given time period, with majority of the patents filed / granted in the past two years.
- In addition to industry players, several academic organizations, such as Stanford University and INSERM have also filed patents related to fc fusion therapeutics.
- The high value patents focus on the modification of Fc region with different fusion molecules, such as receptor ECD, enzyme and peptide.
- Published scientific literature is indicative of the ongoing pace of research in this field; the focus of past and ongoing studies seems to be fixated on the development of anti-cancer therapeutics.
- The past few years have seen a steady rise in the number of publications related to fc fusion therapeutics; these include articles highlighting the type of biological moieties fused with the Fc region of the antibodies.
- Majority (~70%) of the published articles are related to the research studies focused on evaluating the therapeutic potential of Fc fusion proteins across various oncological and blood disorders.
- Articles related to Fc fusion therapeutics have been published in several high impact journals; however, PLoS One and MAbs have emerged as the key journals with over 30 articles.
- With multiple pipeline candidates in the mid to late stages of development, these interventions are primarily targeting oncological disorders; majority of these are Fc protein engineered antibodies.
- A number of marketed drugs, such as Fasenra, Gazyva, Margenza and Skyrizi, are now being evaluated for their efficacy across other oncological indications as well.
- Majority (~60%) of the late-stage candidates are targeting oncological indications, including solid tumors, non-Hodgkin lymphoma, and non-small cell lung cancer.
- Driven by an increasing number of late-stage therapies in the pipeline, the future opportunity, in terms of revenues, is anticipated to grow at an annualized rate of nearly 10%.
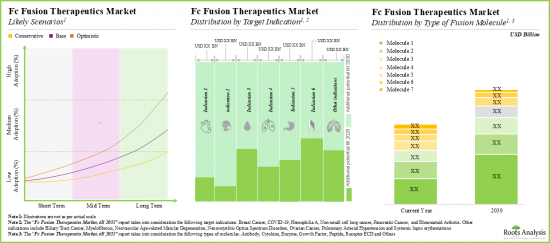
- The estimated market opportunity is expected to be well-distributed across different types of therapies, target indications, type of fusion molecules, routes of administration and key geographic regions.
Example Players in the Drug Discovery Platforms Market
- Abzena
- Creative Biolabs
- Distributed Bio (Subsidiary of Charles River)
- ImmunoPrecise Antibodies
- IONTAS
- LakePharma
- Leading Biology
- Sino Biological
- XOMA
GLOBAL FC FUSION PROTEIN MARKET
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global Fc fusion protein market, focusing on key market segments, including [A] target indications, [B] type of fusion molecule, [C] route of administration and [D] key geographical regions.
- Market Landscape: A comprehensive evaluation of Fc fusion therapeutics, considering various parameters, such as [A] phase of development of lead candidates, [B] type of fusion molecule, [C] target gene, [D] therapeutic area(s), [E] target disease indication(s), [F] type of therapy, [G] route of administration and [H] dosing frequency. Additionally, a detailed evaluation of the drug developer(s), based on [A] year of establishment, [B] company size, and [C] location of headquarters.
- Company Profiles: In-depth profiles of companies engaged in the development of Fc fusion therapeutics, focusing on [A] company overview, [B financial information (if available), [C] product portfolio and [D] recent developments and an informed future outlook.
- Clinical Trial Analysis: A detailed analysis of clinical studies of various Fc fusion therapeutics, based on several relevant parameters, such as [A] trial registration year, [B] trial phase, [C] study design, [D] type of masking, [E] type of intervention model, [F] emerging focus area, [G] leading industry sponsors / collaborators, [H] popular indications, [I] popular interventions and [J] regional distribution of trials.
- Grant Analysis: An in-depth analysis of grants that have been awarded to research institutes engaged in conducting projects related to Fc fusion therapeutics, based on various relevant parameters, such as [A] year of grant award, [B] amount awarded, [C] administering institute center, [D] support period, [E] type of grant application, [F] purpose of grant award, [G] activity code and [H] emerging focus areas.
- Publication Analysis: A comprehensive analysis of close to 1,135 peer-reviewed scientific articles related to Fc fusion therapeutics, based on [A] year of publication, [B] emerging focus areas, [C] target therapeutic area, [D] leading authors and [E] key journals.
- Patent Analysis: An in-depth analysis of patents filed / granted related to Fc fusion therapeutics, based on various relevant parameters, such as [A] publication year, [B] geography, [C] CPC symbols, [D] emerging focus areas, [E] type of applicant, [F] leading industry players and [G] patent valuation analysis.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in this domain, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] focus area, [D] type of partner, [E] most active players (in terms of the number of partnerships signed) and [F] geographical distribution of partnership activity.
- Case Study: A detailed discussion on the Fc protein engineered and glycoengineered antibodies that are either marketed or being developed based on multiple of relevant parameters, such as [A] phase of development, [B] target disease indication, [C] therapeutic area, [D] type of Fc engineering, [E] impact of Fc engineering, [F] route of administration and [G] type of therapy.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Overview of Fc Fusion Therapeutics
- 3.2. Components of Fc Fusion Therapeutics
- 3.3. Mechanism of Action
- 3.4. Types of Fc Fusion Therapeutics
- 3.4.1. Antibody-based Fc Fusion Therapeutics
- 3.4.2. Cytokine-based Fc Fusion Therapeutics
- 3.4.3. Enzyme-based Fc Fusion Therapeutics
- 3.4.4. Peptide-based Fc Fusion Therapeutics
- 3.4.5. Receptor ECD-based Fc Fusion Therapeutics
- 3.5. Applications of Fc Fusion Therapeutics
- 3.6. Advantages of Fc Fusion Therapeutics over Other Biological Moieties
- 3.7. Future Perspectives
4. PIPELINE REVIEW: MARKETED AND CLINICAL DRUGS
- 4.1. Analysis Methodology and Key Parameters
- 4.2. Fc Fusion Therapeutics: Drug Pipeline
- 4.3. Fc Fusion Therapeutics: Pipeline Analysis
- 4.3.1. Analysis by Phase of Development
- 4.3.2. Analysis by Type of Fusion Molecule
- 4.3.3. Analysis by Target Gene
- 4.3.4. Analysis by Therapeutic Area(s)
- 4.3.5. Analysis by Target Disease Indication(s)
- 4.3.6. Analysis by Type of Therapy
- 4.3.7. Analysis by Route of Administration
- 4.3.8. Analysis by Dosing Frequency
- 4.4. Fc Fusion Therapeutics: List of Drug Developers
- 4.4.1. Analysis by Year of Establishment
- 4.4.2. Analysis by Company Size
- 4.4.3. Analysis by Location of Headquarters
- 4.4.4. Leading Developers
- 4.4.5. Grid Analysis: Distribution by Phase of Development, Company Size and Location of Headquarters
5. COMPANY PROFILES
- 5.1. Chapter Overview
- 5.2. Alphamab Oncology
- 5.2.1. Company Overview
- 5.2.2. Financial Information
- 5.2.3. Product Portfolio
- 5.2.4. Recent Developments and Future Outlook
- 5.3. Amgen
- 5.3.1. Company Overview
- 5.3.2. Financial Information
- 5.3.3. Product Portfolio
- 5.3.4. Recent Developments and Future Outlook
- 5.4. Acceleron Pharmaceuticals
- 5.4.1. Company Overview
- 5.4.2. Financial Information
- 5.4.3. Product Portfolio
- 5.4.4. Recent Developments and Future Outlook
- 5.5. Bristol Myers Squibb
- 5.5.1. Company Overview
- 5.5.2. Financial Information
- 5.5.3. Product Portfolio
- 5.5.4. Recent Developments and Future Outlook
- 5.6. Sanofi
- 5.6.1. Company Overview
- 5.6.2. Financial Information
- 5.6.3. Product Portfolio
- 5.6.4. Recent Developments and Future Outlook
6. CLINICAL TRIAL ANALYSIS
- 6.1. Analysis Methodology and Key Parameters
- 6.2. Fc Fusion Therapeutics: List of Clinical Trials
- 6.2.1. Analysis by Trial Registration Year
- 6.2.2. Analysis by Trial Phase
- 6.2.3. Analysis by Study Design
- 6.2.4. Analysis by Type of Masking
- 6.2.5. Analysis by Type of Intervention Model
- 6.2.6. World Cloud: Emerging Focus Areas
- 6.2.7. Analysis by Trial Registration Year and Geography
- 6.2.8. Analysis by Type of Sponsor
- 6.2.9. Leading Industry Players: Analysis by Number of Trials Registered
- 6.2.10. Leading Non-Industry Players: Analysis by Number of Trials Registered
- 6.2.11. Popular Indications: Analysis by Number of Registered Trials
- 6.2.12. Popular Interventions: Analysis by Number of Registered Trials
- 6.2.13. Geographical Analysis by Number of Registered Trials
- 6.2.14. Geographical Analysis by Number of Patients Enrolled
7. ACADEMIC GRANT ANALYSIS
- 7.1. Analysis Methodology and Key Parameters
- 7.2. Fc Fusion Therapeutics: Analysis of Academic Grants
- 7.2.1. Analysis by Year of Grant Award
- 7.2.2. Analysis by Amount Awarded
- 7.2.3. Analysis by Administering Institute Center
- 7.2.4. Analysis by Support Period
- 7.2.5. Analysis by Type of Grant Application
- 7.2.6. Analysis by Purpose of Grant Award
- 7.2.7. Analysis by Activity Code
- 7.2.8. Word Cloud Analysis: Emerging Focus Areas
- 7.2.9. Popular NIH Departments: Analysis by Number of Grants
- 7.2.10. Prominent Program Officers: Analysis by Number of Grants
- 7.2.11. Popular Recipient Organizations: Analysis by Number of Grants
8. PUBLICATION ANALYSIS
- 8.1. Analysis Methodology and Key Parameters
- 8.2. Fc Fusion Therapeutics: Recent Publications
- 8.3. Analysis by Year of Publication
- 8.4. Word Cloud Analysis: Emerging Focus Areas
- 8.5. Analysis by Target Therapeutic Area
- 8.6. Leading Authors: Analysis by Number of Publications
- 8.7. Key Journals: Analysis by Number of Publications
9. PATENT ANALYSIS
- 9.1. Analysis Methodology and Key Parameters
- 9.2. Fc Fusion Therapeutics: Patent Analysis
- 9.2.1. Analysis by Publication Year
- 9.2.2. Analysis by Type of Patent
- 9.2.3. Analysis by Geographical Location
- 9.2.4. Analysis by Patent Age
- 9.2.5. Analysis by CPC Symbols
- 9.2.6. Word Cloud Analysis: Emerging Focus Areas
- 9.2.7. Leading Patent Assignees: Analysis by Number of Patents
- 9.2.8. Leading Industry Players: Analysis by Number of Patents
- 9.2.9. Leading Non-Industry Players: Analysis by Number of Patents
- 9.2.10. Fc Fusion Therapeutics: Patent Benchmarking Analysis
- 9.2.11. Fc Fusion Therapeutics: Patent Valuation Analysis
10. PARTNERSHIPS AND COLLABORATIONS
- 10.1. Analysis Methodology and Key Parameters
- 10.2. Partnership Models
- 10.3. Fc Fusion Therapeutics: List of Partnerships and Collaborations
- 10.3.1. Analysis by Year of Partnership
- 10.3.2. Analysis by Type of Partnership
- 10.3.3. Analysis by Type of Partnership and Type of Fusion molecule
- 10.3.4. Analysis by Year of Partnership and Type of Partner
- 10.3.5. Analysis by Type of Partnership and Type of Partner
- 10.3.6. Most Active Players: Analysis by Number of Partnerships
- 10.3.7. Regional Analysis
- 10.3.7.1. Intercontinental and Intracontinental Agreements
11. MARKET SIZING AND OPPORTUNITY ANALYSIS
- 11.1. Forecast Methodology and Key Assumptions
- 11.2. Global Fc Fusion Therapeutics Market, Till 2035
- 11.3. Global Fc Fusion Therapeutics Market, Till 2035: Distribution by Target Indication
- 11.4. Global Fc Fusion Therapeutics Market, Till 2035: Distribution by Type of Fusion Molecule
- 11.5. Global Fc Fusion Therapeutics Market, Till 2035: Distribution by Type of Therapy
- 11.6. Global Fc Fusion Therapeutics Market, Till 2035: Distribution by Route of Administration
- 11.7. Global Fc Fusion Therapeutics Market, Till 2035: Distribution by Geography
- 11.8. Fc Fusion Therapeutics: Individual Product Sales Forecasts
- 11.8.1. ABP 938 (Amgen)
- 11.8.2. Alprolix(R) (Sanofi)
- 11.8.3. AnBaiNuo(R) (Hisun Pharmaceuticals)
- 11.8.4. Arcalyst(R) (Kiniska Pharmaceuticals)
- 11.8.5. BIVV001 (Sanofi)
- 11.8.6. CD24Fc (Merck)
- 11.8.7. Eloctate(R) (Biogen)
- 11.8.8. Eylea(TM) (Regeneron Pharmaceuticals)
- 11.8.9. FRSW107 (Zhengzhou Gensciences)
- 11.8.10. KN035 (Alphamab Oncology)
- 11.8.11. KN046 (Alphamab Oncology)
- 11.8.12. Lumitin(R) (Chengdu Kanghong Biotech)
- 11.8.13. Reblozyl(R) (Bristol-Myers Squibb)
- 11.8.14. RyzneutaTM (Evive Biotech)
- 11.8.15. Strensiq(R) (AstraZeneca)
- 11.8.16. Telitacicept (RemeGen)
12. CASE STUDY: FC PROTEIN ENGINEERED AND GLYCOENGINEERED ANTIBODIES
- 12.1. Fc Protein Engineered and Glycoengineered Antibodies: Drug Pipeline
- 12.1.1. Analysis by Phase of Development
- 12.1.2. Analysis by Target Disease Indication
- 12.1.3. Analysis by Therapeutic Area
- 12.1.4. Analysis by Type of Fc Engineering
- 12.1.5. Analysis by Impact of Fc Engineering
- 12.1.6. Analysis by Route of Administration
- 12.1.7. Analysis by Type of Therapy
- 12.2. Fc Protein Engineered and Glycoengineered Antibodies: List of Developers
- 12.2.1. Analysis by Year of Establishment
- 12.2.2. Analysis by Company Size
- 12.2.3. Analysis by Location of Headquarters















