
|
시장보고서
상품코드
1771303
세포 리프로그래밍 시장 : 업계 동향 및 예측 - 기술 유형별, 유래 세포 유형별, 용도 유형별, 주요 지역별Cell Reprogramming Market: Industry Trends and Global Forecasts - Distribution by Type of Technology, Type of Source Cell, Type of Application and Key Geographical Regions |
||||||
세계의 세포 리프로그래밍 시장 : 개요
세계의 세포 리프로그래밍 시장 규모는 올해 11억 6,000만 달러에 달했습니다. 이 시장은 예측 기간 동안 8%의 CAGR로 확대될 것으로 예측되고 있습니다.
시장 세분화 및 기회 분석은 다음 매개변수로 세분화됩니다.
기술 유형별
- 센다이 바이러스 기반 리프로그래밍
- mRNA 리프로그래밍
- 에피솜 리프로그래밍
- 기타 리프로그래밍 기술
유래 세포 유형별
- 섬유아세포
- 말초 혈액 단핵 세포
- 불특정체세포
- 기타
용도 유형별
- 연구
- 치료
주요 지역별
- 북미
- 유럽
- 아시아태평양 및 기타 지역
세계의 세포 리프로그래밍 시장 : 성장 및 동향
수년간 줄기세포 생물학과 재생의료 개발로 다양한 줄기세포를 이용한 치료법이 발견 및 개발되어 왔습니다. 그러나 복잡한 제조 과정, 건강한 세포 기증자의 부족, 기증자 및 레시피엔트의 하플로타입 불일치와 관련된 우려 등 세포 치료의 개발과 사용에 대한 과제는 여전히 남아 있습니다. 또한 인간 줄기세포를 연구 목적으로 사용하는 것에는 몇 가지 윤리적 장벽이 있습니다. 그 결과 앞서 언급한 문제를 극복하고 안전하고 효과적인 세포 기반 치료 개입을 개발하는 수단 중 하나로 세포 리프로그래밍의 개념이 부각되어 왔습니다.
바이오테크놀러지 분야의 기술 개발과 재생의료 관련 시장개척에 힘입어 세계 많은 연구그룹이 현재 세포를 초기화하는 혁신적인 방법을 개발하고 있습니다. 게다가 다양한 신흥 기업이나 대학의 스핀오프 기업이, 이 새로운 치료 분야의 개척자로서 대두하고 있어, 향후 수년간도 연구의 기세를 유지할 것으로 예상되고 있는 것은 주목할 만합니다.
세계의 세포 리프로그래밍 시장 : 주요 인사이트
이 보고서는 세계의 세포 리프로그래밍 시장의 현황을 파악하고 업계 내 잠재적 성장 기회를 확인하고 있습니다. 본 보고서의 주요 조사 결과는 다음과 같습니다.
- 세포 리프로그래밍의 진보는 많은 선진적인 치료 및 연구 용도에 대한 길을 열어, 그 결과, 업계 및 비업계의 서비스 제공업체 기업에 유리한 비즈니스 기회를 가져오고 있습니다
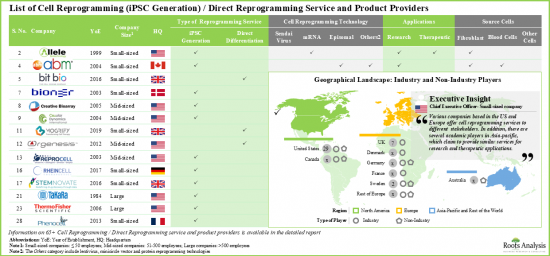
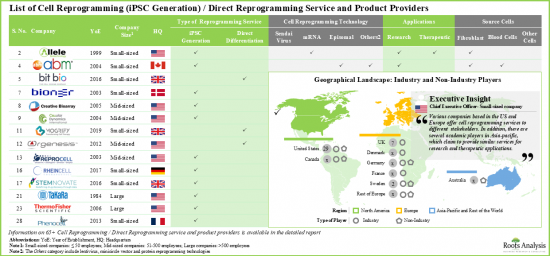
- 30사 이상의 기업이 세포 리프로그래밍의 서비스 및 제품을 제공하고 있다고 주장하고 있습니다. 이 중 70% 근처가 소규모 기업(종업원 50명 미만)입니다.
- 현재, 서비스 제공업체의 대부분(-75%)은 에피솜 초기화 기술을 이용한 iPSC 제작 서비스를 제공하고 있어 다양한 유형의 세포를 취급할 수 있다고 주장하고 있습니다. 여기에 이은 것이 직접 초기화 서비스를 제공하는 기업(30%)입니다.
- 이해관계자의 대부분이 iPS 세포의 제작에 에피솜 초기화법을 사용하고 있다고 주장하고 있는 것은 주목할 가치가 있습니다. 다른 일반적인 세포 리프로그래밍 접근법에는 센다이 바이러스와 mRNA 기반 기술이 포함됩니다.
- 다수의 기업이 iPS 세포의 제작에 2개 이상의 기술을 사용하고 있다고 주장하고 있습니다. 그 예로는 Applied StemCell, Applied Biological Materials, Creative Bioarray, Lonza, Stemnovate 등을 들 수 있습니다.
- 입수 용이성, 증식 속도의 속도, 세포의 견고성 등, 섬유아세포와 관련된 다양한 이점을 고려하면, 이들 세포는 세포 리프로그래밍에 있어서 최대(40% 이상) 이용되고 있습니다.
- 복수의 중소기업이 존재하는 것이 특징으로, 세포 리프로그래밍의 상황은 다양한 지역에 잘 분산되어 있어, 참가 기업은 고객의 요구에 부응하기 위해서 복수의 시설을 설립하고 있습니다.
- 북미는 세포 리프로그래밍의 주요 서비스 거점입니다. 세포 리프로그래밍에 종사하는 기업의 55% 이상이 북미에 본사를 두고 있습니다. 그 다음은 유럽에 본사를 두는 기업(45%)입니다.
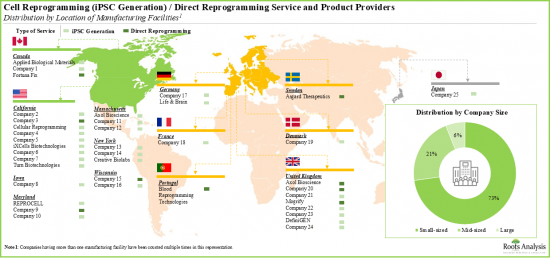
- 유럽의 주요 시설에는 영국, 독일, 덴마크, 스위스, 프랑스, 포르투갈, 스웨덴이 포함되어 있습니다. 이해 관계자는, 각각의 세포 리프로그래밍 서비스의 포트폴리오를 충실시켜, 그 각각의 향후 업계에서 경쟁력을 유지하기 위해서, 적극적으로 능력을 확대하고 있습니다.
- 이 분야에 대한 관심 증가는 과거에 체결된 다양한 유형의 세포를 포함한 파트너십의 수에 반영되어 있습니다.
- 2016년 이후, 제휴 활동은 CAGR 7%로 증가하고 있습니다. 줄기세포에 대한 수요 증가에 따라 산학 양측의 진입 기업이 세포 리프로그래밍을 위해 여러 제휴를 맺을 가능성이 높습니다.
- 기존 기업도 신규 참가 기업도 최근 몇 년간 여러 전략적 제휴를 맺고 있습니다. 거래의 대부분(30%이상)은 주로 iPS 세포의 제작을 목적으로 한 것으로, 고객의 요구에 따라 한층 더 차별화가 도모되고 있습니다.
- 많은 기업들이 세포 초기화와 관련하여 여러 계약을 맺고 있습니다. 4건 이상의 계약을 맺은 기업의 예로는 Cellaria, BlueRock Therapeutics, FUJIFILM Cellular Dynamics, STEMCELL Technologies 등이 있습니다.
- 최근 몇 년간 다양한 유형의 줄기세포 치료를 평가하는 540개 이상의 임상 시험이 시작되었습니다. 이것은, iPS 세포 제작과 직접 리프로그래밍 전략에 대한 수요의 고조를 강조하고 있습니다.
- 이러한 임상 시험의 70% 이상은 중소기업이 스폰서가 되어 실시되고 있어 줄기세포 치료의 가능성을 추구하고 있습니다.
- 줄기세포 치료의 개발에 종사하는 300이상의 이해관계자를 조사한 결과, 세포리프로그래밍 서비스 제공업체에 있어서 전략적 파트너가 될 것 같은 기업이, 다양한 지역에 걸쳐 존재하는 것이 밝혀지고 있습니다.
- 이 시장은 2030년까지 연평균 복합 성장률(CAGR) 8%를 보일 것으로 예측되고 있으며, 그 기회는 다양한 유형의 기술, 용도 분야, 주요 지역에 걸쳐 분산하고 있을 가능성이 높습니다.
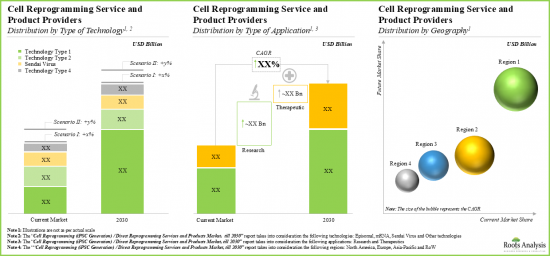
- 현재, 서비스 수익의 대부분은 에피솜 리프로그래밍 기술을 포함한 프로젝트로부터 만들어지고 있습니다.
- 2030년에는 에피솜 초기화에 초점을 맞춘 프로젝트가 전체의 40% 이상을 차지할 것으로 예상되며, 이에 센다이 바이러스 기반 초기화(30%)와 mRNA(25%)를 포함한 프로젝트로부터의 수익이 계속될 것으로 예측됩니다.
세포 리프로그래밍 시장의 진출기업 예
- Allele Biotechnology
- ALSTEM
- Applied Biological Materials
- Axol Bioscience
- Creative Bioarray
- DefiniGEN
- FUJIFILM Cellular Dynamics
- Lonza
- Mogrify
- REPROCELL
- Stemnovate
- Thermo Fisher Scientific
본 보고서에서는 세계의 세포 리프로그래밍 시장에 대해 조사했으며, 시장 개요와 함께 기술 유형별, 유래 세포 유형별, 용도 유형별 동향, 지역별 동향 및 시장 진출기업 프로파일 등의 정보를 제공합니다.
목차
제1장 서문
제2장 주요 요약
제3장 서문
- 장의 개요
- 줄기세포 개요
- 세포 리프로그래밍 서문
- 세포 리프로그래밍 용도
- 주요 성장 요인 및 억제요인
제4장 현재 시장 상황
- 장의 개요
- 세포 리프로그래밍 서비스 및 제품 프로바이더 : 업계 진출기업 일람
- 세포 리프로그래밍 서비스 및 제품 프로바이더 : 업계외 진입기업 목록
- 세포 리프로그래밍 : 관련 제품 공급자 목록
제5장 기업 경쟁력 분석
- 장의 개요
- 전제 및 주요 파라미터
- 조사 방법
- 경쟁력 분석 : iPSC 생성 서비스 및 제품 공급자
- 경쟁력 분석 : 직접 프로그래밍 서비스 및 제품 공급자
제6장 기업 프로파일
- 장의 개요
- Allele Biotechnology
- ALSTEM
- Applied Biological Materials
- Axol Bioscience
- Creative Bioarray
- DefiniGEN
- FUJIFILM Cellular Dynamics International
- Lonza
- Mogrify
- REPROCELL
- Stemnovate
- Thermo fisher Scientific
제7장 사례 연구 : 줄기세포 치료 개발에 있어서 임상 시험 활동
- 장의 개요
- 범위 및 조사 방법
- 줄기세포 요법 : 임상 시험 분석
제8장 파트너십 및 협업
- 장의 개요
- 범위 및 조사 방법
- 세포 리프로그래밍 서비스 및 제품 시장 : 파트너십 및 협업 목록
제9장 파트너십 가능성
- 장의 개요
- 범위 및 조사 방법
- 채점 기준 및 주요 전제
- 줄기세포 요법 개발자 : 세포 리프로그래밍 서비스 및 제품 프로바이더의 유망한 파트너
- 줄기세포 요법 수탁 제조자 : 세포 리프로그래밍 서비스 및 제품 프로바이더의 유망한 파트너
제10장 시장 예측
- 장의 개요
- 범위 및 조사 방법
- 예측 조사 방법 및 주요 전제조건
- 세계의 세포 리프로그래밍 서비스 및 제품 시장(-2035년)
- 세계의 세포 리프로그래밍 서비스 및 제품 시장(-2035년) : 기술 유형별
- 세계의 세포 리프로그래밍 서비스 및 제품 시장(-2035년) : 유래 세포 유형별
- 세계의 세포 리프로그래밍 서비스 및 제품 시장(-2035년) : 용도 유형별
- 세계의 세포 리프로그래밍 서비스 및 제품 시장(-2035년) : 주요 지역별 분포
제11장 주요 인사이트
제12장 결론
제13장 부록 1 : 표 형식 데이터
제14장 부록 2 : 기업 및 단체 일람
AJY 25.07.21GLOBAL CELL REPROGRAMMING MARKET: OVERVIEW
As per Roots Analysis, the global cell reprogramming market valued at USD 1.16 billion in the current year is anticipated to grow at a CAGR of 8% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Technology
- Sendai Virus-based Reprogramming
- mRNA Reprogramming
- Episomal Reprogramming
- Other Reprogramming Technologies
Type of Source Cell
- Fibroblasts
- Peripheral Blood Mononuclear Cells
- Unspecified Somatic Cells
- Other Cells
Type of Application
- Research
- Therapeutic
Key Geographical Regions
- North America
- Europe
- Asia-Pacific and Rest of the World
GLOBAL CELL REPROGRAMMING MARKET: GROWTH AND TRENDS
Progress in stem cell biology and regenerative medicine over the years has led to the discovery and development of various stem cell-based therapies. However, prevalent challenges concerning the development and use of cell therapies persist, including complex production process, scarcity of healthy cell donors, and concerns associated with donor-recipient haplotype mismatch. Moreover, there are still several ethical barriers when it comes to using human stem cells for research purposes. Consequently, the concept of cell reprogramming has emerged as one of the means to overcome the aforementioned issues and develop safe and effective cell-based therapeutic interventions.
Empowered by technical developments in the field of biotechnology and the market opportunity associated with regenerative medicine, a number of research groups across the world are presently developing innovative ways to reprogram cells. Further, it is worth noting that various start-ups and university spin-offs have emerged as pioneers in this emerging field of therapeutics and are anticipated to maintain the research momentum in the coming years as well.
GLOBAL CELL REPROGRAMMING MARKET: KEY INSIGHTS
The report delves into the current state of global cell reprogramming market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Advances in cell reprogramming have paved way for a plethora of advanced therapeutic and research applications, resulting in lucrative business opportunities for industry and non-industry service provider firms.
- More than 30 companies claim to offer cell reprogramming services and products. Of these, close to 70% are small firms (having less than 50 employees).
- Presently, the majority (~75%) of the service providers claim to offer iPSC generation services, using episomal reprogramming technologies, and are capable of working with various types of cells. This is followed by the companies offering direct reprogramming services (30%).
- It is worth mentioning that majority of stakeholders claim to be using episomal reprogramming for generating iPSCs; other popular cell reprograming approaches include Sendai virus and mRNA-based technologies.
- Numerous companies claim to use more than two technologies for the generation of iPSCs; examples include Applied StemCell, Applied Biological Materials, Creative Bioarray, Lonza and Stemnovate.
- Given the various advantages associated with fibroblasts, such as easy availability, faster proliferation rate and cell robustness, these cells have found maximum (>40%) usage in cell reprogramming.
- Featuring the presence of several small and mid-sized firms, the cell reprogramming landscape is well distributed across various regions; players have established multiple facilities to cater to the needs of their clients.
- North America is a key services hub for cell reprogramming. More than 55% of companies engaged in cell reprogramming are headquartered in North America. This is followed by players based in Europe (45%).
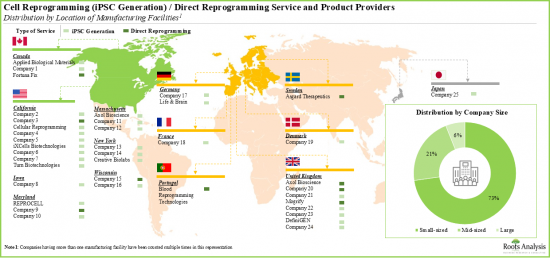
- Prominent facilities within Europe include the UK, Germany, Denmark, Switzerland, France, Portugal and Sweden. Stakeholders are actively expanding their capabilities in order to enhance their respective cell reprogramming service portfolios and, thereby, maintain a competitive edge in this upcoming industry.
- The rising interest in this field is reflected in the number of partnerships, involving a varied range of cell types, inked in the recent past; the maximum partnering activity has been observed in the US.
- Since 2016, the partnership activity has increased at a CAGR of 7%; with the increasing demand for stem cells, both industry and academic players are likely to enter into multiple alliances for cell reprogramming.
- Both established players and new entrants have forged several strategic partnerships in the recent past; majority (>30%) of the deals have primarily been inked for generations of iPSCs, which are further differentiated based on the client's requirement.
- A number of companies have signed multiple deals for cell reprogramming; examples of the firms that have signed over four deals include Cellaria, BlueRock Therapeutics, FUJIFILM Cellular Dynamics and STEMCELL Technologies.
- In the last few years, over 540 clinical trials, evaluating various types of stem cell therapies have been initiated; this highlights the rising demand for iPSC generation and direct reprogramming strategies.
- >70% of these trials were / are being sponsored by small / mid-sized companies to exploit the potential of stem cell therapies to cater to the unmet need in this domain.
- An evaluation of more than 300+ stakeholders engaged in the development of stem cell therapies reveals several likely strategic partners for cell reprogramming service providers, across different geographical regions.
- The market is anticipated to grow at a CAGR of 8% till 2030, and the opportunity is likely to be distributed across different types of technologies, applications and key geographical regions.
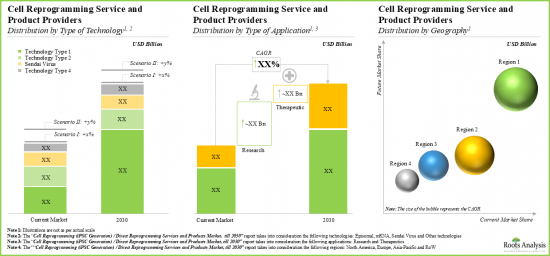
- Presently, the majority share of service revenues is generated from projects involving episomal reprogramming technologies.
- In 2030, episomal reprogramming focused projects are anticipated to contribute over 40% of the overall share; it is expected to be followed by revenues generated from projects involving Sendai virus-based reprogramming (30%) and mRNA (25%).
Example Players in the Cell Reprogramming Market
- Allele Biotechnology
- ALSTEM
- Applied Biological Materials
- Axol Bioscience
- Creative Bioarray
- DefiniGEN
- FUJIFILM Cellular Dynamics
- Lonza
- Mogrify
- REPROCELL
- Stemnovate
- Thermo Fisher Scientific
GLOBAL CELL REPROGRAMMING MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global cell reprogramming market, focusing on key market segments, including [A] type of technology, [B] type of source cell, [C] type of application and [D] key geographical regions.
- Market Landscape: A comprehensive evaluation of cell reprogramming market, considering various parameters, such as [A] year of establishment, [B] company size, [C] geographical location, [D] location of manufacturing facilities, [E] type of service, [F] type of offering, [G] type of technology, [H] type of vector used, [I] source cell for iPSC generation, [J] target indication(s), [K] type of application and [L] additional service(s) offered.
- Company Competitiveness Analysis: A comprehensive company competitive analysis of iPSC generation and direct reprogramming service providers, examining factors, such as [A] supplier strength and [B] service strength.
- Company Profiles: In-depth profiles of the players that offer cell reprogramming services and products, focusing on [A] company overview, [B] financial information (if available), [C] details on cell reprogramming approaches, [D] types of cell(s) utilized and differentiated, [E] target indication(s), [F] other drug discovery services offered and [G] recent developments and an informed future outlook.
- Clinical Trial Analysis: An insightful analysis more than 540 completed, ongoing and planned clinical studies of various stem cell therapies, based on several parameters, such as [A] trial registration year, [B] phase of development, [C] study design, [D] current trial status, [E] leading industry sponsors, [F] study focus, [G] type of stem cells, [H] target indication(s), [I] target therapeutic area(s), [J] enrolled patient population and [K] regional distribution of trials.
- Partnerships and Collaborations: An insightful analysis of the deals inked by stakeholders in the cell reprogramming market, based on several parameters, such as [A] year of partnership, [B] type of partnership, [C] type of stem cell, [D] target therapeutic area, [E] application, [F] most active players (in terms of the number of partnerships signed) and [G] geography.
- Likely Partnership Opportunities: A detailed discussion on the stem cell therapy developers / manufacturers that are anticipated to partner with cell reprogramming service and product providers in the foreseen future, based on various relevant parameters, such as [A] company size, [B] type of cell (s), [C] indication counts and [D] existing partnership(s).
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Overview of Stem Cells
- 3.2.1. Classification of Stem Cells
- 3.2.1.1. Based on Source of Stem Cell
- 3.2.1.2. Based on Origin of Stem Cell
- 3.2.1.3. Based on Potency of Stem Cell
- 3.2.2. Routes of Administration of Stem Cell Therapies
- 3.2.3. Applications of Stem Cell Therapies
- 3.2.1. Classification of Stem Cells
- 3.3. Introduction to Cell Reprogramming
- 3.3.1. Cell Reprogramming Approaches and Affiliated Technologies
- 3.4. Applications of Cell Reprogramming
- 3.5. Key Growth Drivers and Constraints
4. CURRENT MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Cell Reprogramming Service and Product Providers: List of Industry Players
- 4.2.1. Analysis by Year of Establishment
- 4.2.2. Analysis by Company Size
- 4.2.3. Analysis by Geographical Location
- 4.2.4. Analysis by Location of Stem Cell Research Facilities
- 4.2.5. Analysis by Type of Service
- 4.2.6. Analysis by Type of Technology
- 4.2.7. Analysis by Target Indication
- 4.2.8. Analysis by Source Cell
- 4.2.9. Analysis by Application Area
- 4.2.10. Analysis by Additional Service(s) Offered
- 4.2.11. Analysis by Type of Offering
- 4.3. Cell Reprogramming Service and Product Providers: List of Non-Industry Players
- 4.3.1. Analysis by Geographical Location
- 4.3.2. Analysis by Type of Technology Used
- 4.3.3. Analysis by Source Cell for iPSC Generation
- 4.3.4. Analysis by Type of Offering
- 4.4. Cell Reprogramming: List of Affiliated Products Providers
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Assumptions and Key Parameters
- 5.3. Methodology
- 5.4. Competitiveness Analysis: iPSC Generation Service and Product Providers
- 5.5. Competitiveness Analysis: Direct Reprogramming Service and Product Providers
6. COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. Allele Biotechnology
- 6.2.1. Company Overview
- 6.2.2. Recent Developments and Future Outlook
- 6.3. ALSTEM
- 6.3.1. Company Overview
- 6.3.2. Recent Developments and Future Outlook
- 6.4. Applied Biological Materials
- 6.4.1. Company Overview
- 6.4.2. Recent Developments and Future Outlook
- 6.5. Axol Bioscience
- 6.5.1. Company Overview
- 6.5.2. Recent Developments and Future Outlook
- 6.6. Creative Bioarray
- 6.6.1. Company Overview
- 6.6.1.1. Recent Developments and Future Outlook
- 6.6.1. Company Overview
- 6.7. DefiniGEN
- 6.7.1. Company Overview
- 6.7.2. Recent Developments and Future Outlook
- 6.8. FUJIFILM Cellular Dynamics International
- 6.8.1. Company Overview
- 6.8.2. Recent Developments and Future Outlook
- 6.9. Lonza
- 6.9.1. Company Overview
- 6.9.2. Recent Developments and Future Outlook
- 6.10. Mogrify
- 6.10.1. Company Overview
- 6.10.2. Recent Developments and Future Outlook
- 6.11. REPROCELL
- 6.11.1. Company Overview
- 6.11.1.1. Recent Developments and Future Outlook
- 6.11.1. Company Overview
- 6.12. Stemnovate
- 6.12.1. Company Overview
- 6.12.2. Recent Developments and Future Outlook
- 6.14. Thermo fisher Scientific
- 6.14.1. Company Overview
- 6.14.1.1. Recent Developments and Future Outlook
- 6.14.1. Company Overview
7. CASE STUDY: CLINICAL TRIAL ACTIVITY IN STEM CELL THERAPY DEVELOPMENT
- 7.1. Chapter Overview
- 7.2. Scope and Methodology
- 7.3. Stem Cell Therapies: Clinical Trial Analysis
- 7.3.1. Analysis by Trial Registration Year
- 7.3.2. Analysis by Trial Phase
- 7.3.3. Analysis by Number of Patients Enrolled by Trial Registration Year
- 7.3.4. Analysis by Study Design
- 7.3.5. Analysis by Trial Recruitment Status
- 7.3.6. Analysis by Sponsor / Collaborator
- 7.3.7. Leading Industry Sponsors: Analysis by Number of Registered Trials
- 7.3.8. Analysis by Trial Focus
- 7.3.9. Analysis by Type of Stem Cell
- 7.3.10. Analysis by Therapeutic Area
- 7.3.11. Analysis by Type of Stem Cell and Therapeutic Area
- 7.3.12. Geographical Analysis by Number of Clinical Trials
- 7.3.13. Geographical Analysis by Trial Recruitment Status
- 7.3.14. Geographical Analysis by Enrolled Patient Population
8. PARTNERSHIPS AND COLLABORATIONS
- 8.1. Chapter Overview
- 8.2. Scope and Methodology
- 8.3. Cell Reprogramming Services and Products Market: List of Partnerships and Collaborations
- 8.3.1. Analysis by Year of Partnership
- 8.3.2. Analysis by Type of Partnership
- 8.3.3. Analysis by Year of Partnership and Type of Partner
- 8.3.4. Analysis by Type of Partnership and Type of Partner
- 8.3.5. Analysis by Type of Stem Cell
- 8.3.6. Analysis by Target Therapeutic Area
- 8.3.7. Analysis by Application
- 8.3.8. Most Active Players: Analysis by Number of Partnerships
- 8.3.9. Regional Analysis
- 8.3.10. Intercontinental and Intracontinental Agreements
9. LIKELY PARTNERSHIP OPPORTUNITIES
- 9.1. Chapter Overview
- 9.2. Scope and Methodology
- 9.3. Scoring Criteria and Key Assumptions
- 9.4. Stem Cell Therapy Developers: Likely Partners for Cell Reprogramming Service and Product Providers
- 9.4.1. Likely Partners for Cell Reprogramming Service and Product Providers in North America
- 9.4.2. Likely Partners for Cell Reprogramming Service and Product Providers in Europe
- 9.4.3. Likely Partners for Cell Reprogramming Service and Product Providers in Asia-Pacific and Rest of the World
- 9.5. Stem Cell Therapy Contract Manufacturers: Likely Partners for Cell Reprogramming Service and Product Providers
- 9.5.1. Likely Partners for Cell Reprogramming Service and Product Providers in North America
- 9.5.2. Likely Partners for Cell Reprogramming Service and Product Providers in Europe
- 9.5.3. Likely Partners for Cell Reprogramming Service and Product Providers in Asia-Pacific and Rest of the World
10. MARKET FORECAST
- 10.1. Chapter Overview
- 10.2. Scope and Methodology
- 10.3. Forecast Methodology and Key Assumptions
- 10.4. Global Cell Reprogramming Services and Products Market, Till 2035
- 10.4.1. Global Cell Reprogramming Services and Products Market: Distribution by Type of Technology, Till 2035
- 10.4.1.1. Cell Reprogramming Services and Products Market for Episomal Reprogramming, Till 2035
- 10.4.1.2. Cell Reprogramming Services and Products Market for mRNA Reprogramming, Till 2035
- 10.4.1.3. Cell Reprogramming Services and Products Market for Sendai Virus-based Reprogramming, Till 2035
- 10.4.1.4. Cell Reprogramming Services and Products Market for Other Reprogramming Technologies, Till 2035
- 10.4.2. Global Cell Reprogramming Services and Products Market: Distribution by Type of Source Cell, Till 2035
- 10.4.2.1. Cell Reprogramming Services and Products Market for Fibroblasts, Till 2035
- 10.4.2.2. Cell Reprogramming Services and Products Market for Peripheral Blood Mononuclear Cells, Till 2035
- 10.4.2.3. Cell Reprogramming Services and Products Market for Unspecified Somatic Cells, Till 2035
- 10.4.2.4. Cell Reprogramming Services and Products Market for Other Cells, Till 2035
- 10.4.3. Global Cell Reprogramming Services and Products Market: Distribution by Type of Application, Till 2035
- 10.4.3.1. Cell Reprogramming Services and Products Market for Research, Till 2035
- 10.4.3.2. Cell Reprogramming Services and Products Market for Therapeutic, Till 2035
- 10.4.4. Global Cell Reprogramming Services and Products Market: Distribution by Key Geographical Regions, Till 2035
- 10.4.4.1. Cell Reprogramming Services and Products Market in North America, Till 2035
- 10.4.4.2. Cell Reprogramming Services and Products Market in Europe, Till 2035
- 10.4.4.3. Cell Reprogramming Services and Products Market in Asia-Pacific and Rest of the World, Till 2035
- 10.4.1. Global Cell Reprogramming Services and Products Market: Distribution by Type of Technology, Till 2035
11. EXECUTIVE INSIGHTS
- 11.1. Chapter Overview
- 11.2. Company A
- 11.2.1. Company Snapshot
- 11.2.2. Interview Transcript: Co-Founder and Chief Executive Officer
- 11.3. Company B
- 11.3.1. Company Snapshot
- 11.3.2. Interview Transcript: Chief Scientific Officer














