
|
시장보고서
상품코드
1793323
상처 관리 시장 : 제품별, 상처 유형별, 최종 사용자별, 지역별 예측(-2030년)Wound Care Market by Product (Dressings, Devices, Biological Skin Substitutes, Sutures, Staplers), By Wounds, By End User, and Region -Global Forecast to 2030 |
||||||
세계의 상처 관리 시장 규모는 2025년 222억 2,000만 달러에서 2030년까지 304억 8,000만 달러에 이를 것으로 예측되며, 예측 기간에 CAGR 6.5%의 성장이 예상됩니다.
| 조사 범위 | |
|---|---|
| 조사 대상 연도 | 2024-2030년 |
| 기준연도 | 2024년 |
| 예측 기간 | 2025-2030년 |
| 단위 | 10억 달러 |
| 부문 | 제품, 상처 유형, 최종 사용자 |
| 대상 지역 | 북미, 유럽, 아시아태평양, 라틴아메리카, 중동 및 아프리카 |
이는 당뇨병 환자 증가, 외상성 부상 및 화상 부상 사례 증가, 고령 인구 증가 등이 시장 성장을 촉진하기 때문입니다. 또한 고급 상처 관리 제품의 위험이 시장 성장을 제약할 것으로 예상됩니다.

최종 사용자별 상처 관리 시장 부문 중 병원 및 클리닉 부문은 예측 기간 동안 가장 큰 시장 점유율을 차지할 것으로 예상됩니다.
최종 사용자별로 시장은 병원 및 클리닉, 재택 치료, 장기 요양 시설 및 기타 최종 사용자로 세분화됩니다. 2024년 기준 병원 및 클리닉이 시장 점유율의 가장 큰 부분을 차지합니다. 이 우위는 당뇨병성 발 궤양 및 수술 상처의 발생률 증가가 전문적인 의료 처치를 촉진하기 때문입니다. 또한 당뇨병 및 혈관 질환과 같은 만성 질환을 가진 고령 인구의 증가로 인해 상처 관리 목적으로 병원 방문이 증가하고 있습니다. 병원과 전문 치료 센터는 경험이 풍부한 의료 인력, 표준화된 치료 프로토콜, 다양한 상처 관리 제품을 갖추고 있어 효율적이고 효과적인 치료를 가능하게 합니다. 상처 관리 결과 개선과 환자 회복에 대한 지속적인 강조는 이 부문의 선도적 위치를 더욱 강화하고 있습니다.
아시아태평양은 상처 관리 시장 가장 빠르게 성장하는 지역입니다.
전 세계 상처 관리 시장은 북미, 유럽, 아시아태평양, 라틴 아메리카, 중동 및 아프리카 등 5개 부문으로 세분화됩니다. 아시아태평양 상처 관리 시장은 고령 인구 증가와 만성 상처를 유발하는 생활습관 질환의 확산으로 인해 성장하고 있습니다. 품질 높은 의료 서비스에 대한 수요 증가와 병원 인프라 및 의료 기술에 대한 투자 확대는 고급 상처 관리 제품의 채택을 촉진하고 있습니다. 또한 인도, 태국, 싱가포르 등 국가에서 의료 관광이 급증하면서 수술 건수가 촉진되고 있으며, 이는 수술 후 상처 관리 수요를 더욱 높이고 있습니다. 해당 지역 의료 제산업체들이 치료 결과 개선과 회복 시간 단축에 집중함에 따라 시장은 강력한 성장을 앞두고 있으며, 아시아태평양 지역은 예측 기간 동안 가장 높은 CAGR을 기록할 것으로 예상됩니다.
본 보고서는 세계의 상처 관리 시장에 대한 조사 분석을 통해 주요 촉진요인과 억제요인, 경쟁 구도, 미래 동향 등의 정보를 제공합니다.
목차
제1장 서론
제2장 조사 방법
제3장 주요 요약
제4장 중요한 지견
- 상처 관리 시장 개요
- 아시아태평양 상처 관리 시장 점유율 : 최종 사용자별, 국가별
- 상처 관리 시장 : 주요 국가별
- 상처 관리 시장 : 지역 구성(2023-2030년)
제5장 시장 개요
- 소개
- 시장 역학
- 성장 촉진요인
- 억제요인
- 기회
- 과제
- 고객사업에 영향을 주는 동향 및 혼란
- 가격 설정 분석
- 평균 판매 가격 동향 : 제품별
- 상처 관리 제품의 평균 판매 가격 동향 : 주요 상처별, 주요 기업별
- 평균 판매 가격 동향 : 지역별
- 밸류체인 분석
- 공급망 분석
- 생태계 분석
- 투자 및 자금조달 시나리오
- 기술 분석
- 주요 기술
- 보완 기술
- 인접 기술
- 특허 분석
- 무역 분석
- 수입 시나리오
- 수출 시나리오
- 주요 컨퍼런스 및 이벤트(2025-2026년)
- 사례 연구 분석
- 사례 연구 1 : 복잡한 상처 치요에 고급 상처 관리 방식 채택
- 사례 연구 2 : 상처 관리에서 새로운 하이드로콜로이드 드레싱 평가
- 사례 연구 3 : 콜로플라스트가 개발한 간단한 3단계 프레임워크를 활용한 치료가 어려운 압박성 궤양의 지원형 자기 관리
- 규제 상황
- 규제 분석
- 규제기관, 정부기관, 기타 조직
- Porter's Five Forces 분석
- 주요 이해관계자와 구매 기준
- 상처 관리 시장에 대한 AI의 영향
- 소개
- 상처 관리 시장에 있어서의 AI 시장 장래성
- 상처 관리 시장의 AI의 영향 : 이용 사례
- AI를 도입하고 있는 주요 기업
- 상처 관리 시장에서 AI의 미래
- 미국 관세의 영향(2025년)
- 소개
- 주요 관세율
- 가격 영향 분석
- 국가, 지역에 미치는 영향
- 최종 이용 산업에 미치는 영향
제6장 상처 관리 시장 : 제품별
- 소개
- 고급 상처 관리 제품
- 수술용 상처 관리 제품
- 기존 상처 관리 제품
제7장 창상 케어 시장 : 상처 유형별
- 소개
- 만성 상처
- 급성 상처
제8장 상처 관리 시장 : 최종 사용자별
- 소개
- 병원 및 진료소
- 재택 케어 환경
- 장기 요양 시설
- 기타 최종 사용자
제9장 상처 관리 시장 : 지역별
- 소개
- 북미
- 북미의 거시경제 전망
- 미국
- 캐나다
- 유럽
- 유럽의 거시 경제 전망
- 독일
- 프랑스
- 영국
- 이탈리아
- 스페인
- 러시아
- 기타 유럽
- 아시아태평양
- 아시아태평양의 거시 경제 전망
- 중국
- 일본
- 인도
- 호주
- 한국
- 싱가포르
- 기타 아시아태평양
- 라틴아메리카
- 라틴아메리카의 거시 경제 전망
- 브라질
- 멕시코
- 기타 라틴아메리카
- 중동 및 아프리카
제10장 경쟁 구도
- 개요
- 주요 진입기업의 전략 및 강점(2024년)
- 수익 분석(2022-2024년)
- 시장 점유율 분석(2024년)
- 기업 평가 매트릭스 : 주요 기업(2024년)
- 기업의 평가 매트릭스 : 스타트업/중소기업(2024년)
- 기업 평가 및 재무 지표
- 브랜드/제품 비교
- 경쟁 시나리오
제11장 기업 프로파일
- 주요 기업
- SOLVENTUM
- JOHNSON & JOHNSON SERVICES, INC.(ETHICON)
- SMITH NEPHEW
- CARDINAL HEALTH
- MOLNLYCKE AB
- CONVATEC GROUP PLC
- COLOPLAST GROUP
- ORGANOGENESIS INC.
- PAUL HARTMANN AG
- MIMEDX GROUP, INC.
- OWENS & MINOR
- B. BRAUN SE
- ESSITY AKTIEBOLAG
- INTEGRA LIFESCIENCES CORPORATION
- AVERY DENNISON CORPORATION
- MATIV HOLDINGS, INC.
- BIOVENTUS
- ZIMMER BIOMET
- MEDTRONIC
- BAXTER
- 기타 기업
- LOHMANN & RAUSCHER GMBH & CO. KG
- MEDLINE INDUSTRIES, LP
- DEROYAL INDUSTRIES, INC.
- WINNER MEDICAL CO., LTD.
- ADVANCIS(UK)
- MIL LABORATORIES PVT. LTD.
- PENSAR MEDICAL
- HAROMED BV
- URGO GROUP
- DIRECT HEALTHCARE GROUP
제12장 부록
HBR 25.08.22The global wound care market is projected to reach USD 30.48 billion by 2030 from USD 22.22 billion in 2025, at a CAGR of 6.5% during the forecast period.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2024-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD billion) |
| Segments | Product, Wound type, and End User |
| Regions covered | North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa |
This is due to the increasing rise of diabetic patients, rising cases of traumatic injuries and burn injuries, and the growing geriatric population, which drives market growth. Additionally, the risk of advanced wound care products restraining the market growth.
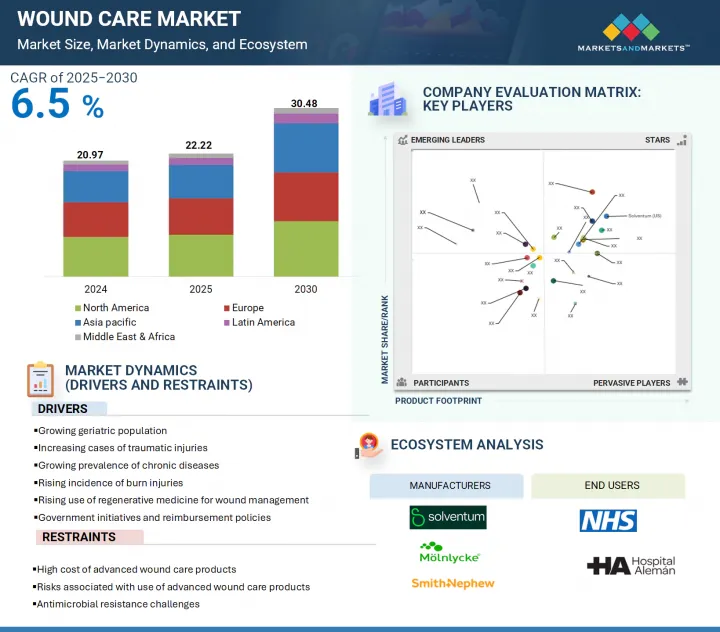
The hospitals and clinics segment of the wound care market, by end user, is expected to hold the largest position during the forecast period.
Based on end user, the market is segmented into hospitals and clinics, home care settings, long-term care facilities, and other end users. In 2024, hospitals and clinics account for the largest share of the market. This dominance is driven by the rising prevalence of diabetic foot ulcers and surgical wounds, which require professional medical attention. Additionally, the aging population with chronic conditions such as diabetes and vascular disorders has led to an increase in hospital visits for wound care. Hospitals and specialized care centers are well equipped with experienced medical personnel, standardized treatment protocols, and a comprehensive range of wound care products, enabling efficient and effective treatment. The continued emphasis on enhancing wound care outcomes and patient recovery further reinforces the leading position of this segment.
Asia Pacific is the fastest-growing region of the wound care market.
The global wound care market is segmented into five segments, namely, North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. The Asia Pacific wound care market is expanding due to a growing aging population and a rise in lifestyle-related diseases that contribute to chronic wounds. Increased demand for quality healthcare and greater investments in hospital infrastructure and medical technologies are boosting the adoption of advanced wound care products. Moreover, the surge in medical tourism across countries like India, Thailand, and Singapore is driving surgical volumes, further increasing the need for post-operative wound care. As healthcare providers in the region focus on improving treatment outcomes and reducing recovery times, the market is poised for robust growth, with Asia Pacific projected to witness the highest CAGR during the forecast period.
A breakdown of the primary participants referred to for this report is provided below:
- By Company Type: Tier 1: 40%, Tier 2: 30%, and Tier 3: 30%
- By Designation: C Level: 27%, Director Level: 18%, and Others: 55%
- By Region: North America: 51%, Europe: 21%, Asia Pacific: 18%, Latin America: 6%, and Middle East & Africa: 4%
Note 1: Companies are classified into tiers based on their total revenue. As of 2023, Tier 1 = >USD 10.00 billion, Tier 2 = USD 1.00 billion to USD 10.00 billion, and Tier 3 = <USD 1.00 billion.
Note 2: C-level primaries include CEOs, CFOs, COOs, and VPs.
Note 3: Other designations include sales managers, marketing managers, business development managers, product managers, distributors, and suppliers.
The major players operating in the wound care market are Solventum (US), Johnson & Johnson Services, Inc. (US), Smith+ Nephew (UK), Convatec Group PLC (UK), Coloplast Group (Denmark), Cardinal Health (US), Molnlycke AB (Sweden), Integra LifeSciences Corporation (US), PAUL HARTMANN AG (Germany), B.Braun SE (Germany), Organogenesis Inc. (US), MIMEDX Group, Inc. (US), Essity Aktlebolag (Sweden), Avery Dennison Corporation (US), Mativ Holdings, Inc. (US), Owens & Minor (US), Zimmer Biomet (US), Bioventus (US), Medtronic (Ireland), and Baxter (US).
Research Coverage
This report studies the wound care market based on product, wound type, end user, and country. The report also studies factors (such as drivers, restraints, opportunities, and challenges) affecting market growth and provides details of the competitive landscape for market leaders. Furthermore, the report analyzes micro markets with respect to their individual growth trends. It forecasts the revenue of the market segments with respect to five major regions (and the respective countries in these regions).
Reasons to Buy the Report
The report will enable established firms as well as entrants/smaller firms to gauge the pulse of the market, which, in turn, would help them to garner a larger market share. Firms purchasing the report could use one or a combination of the strategies mentioned below to strengthen their market presence.
This report provides insights on the following pointers:
- Analysis of key drivers (growing geriatric population, increasing cases of traumatic injuries, growing prevalence of chronic diseases
rising incidence of burn injuries, rising use of regenerative medicine for wound management,
and government initiatives and reimbursement policies), restraints (high cost of advanced wound care products, risks associated with use of advanced wound care products
and antimicrobial resistance challenges), opportunities (Growth opportunities in emerging economies, technological advancement in wound care, and home-care optimized solutions), challenges (lack of trained healthcare professionals, Limited awareness in underdeveloped regions, and data security and privacy concerns)
- Market Penetration: Comprehensive information on the product portfolios offered by the top players in the wound care market
- Product Development/Innovation: Detailed insights on the upcoming trends, R&D activities, and product launches in the wound care market
- Market Development: Comprehensive information on lucrative emerging regions
- Market Diversification: Exhaustive information about new products, growing geographies, and recent developments in the wound care market
- Competitive Assessment: In-depth assessment of market segments, growth strategies, revenue analysis, and products of the leading market players
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION AND REGIONAL SCOPE
- 1.3.2 INCLUSIONS AND EXCLUSIONS
- 1.4 YEARS CONSIDERED
- 1.5 CURRENCY CONSIDERED
- 1.6 STAKEHOLDERS
- 1.7 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Key data from secondary sources
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Primary sources
- 2.1.2.2 Key industry insights
- 2.1.2.3 Key data from primary sources
- 2.1.2.4 Breakdown of primary interviews
- 2.1.1 SECONDARY DATA
- 2.2 MARKET SIZE ESTIMATION
- 2.2.1 BOTTOM-UP APPROACH
- 2.2.1.1 Approach 1: Company revenue estimation approach
- 2.2.1.2 Approach 2: Presentations of companies
- 2.2.1.3 Approach 3: Primary interviews
- 2.2.1.4 Growth forecast
- 2.2.1.5 CAGR projections
- 2.2.2 TOP-DOWN APPROACH
- 2.2.1 BOTTOM-UP APPROACH
- 2.3 MARKET BREAKDOWN AND DATA TRIANGULATION
- 2.4 RESEARCH ASSUMPTIONS
- 2.4.1 STUDY-RELATED ASSUMPTIONS
- 2.4.2 ASSUMPTIONS, BY PARAMETER
- 2.4.3 GROWTH RATE ASSUMPTIONS
- 2.5 RESEARCH LIMITATIONS
- 2.6 RISK ASSESSMENT
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 WOUND CARE MARKET OVERVIEW
- 4.2 ASIA PACIFIC: WOUND CARE MARKET SHARE, BY END USER AND COUNTRY
- 4.3 WOUND CARE MARKET, BY KEY COUNTRY
- 4.4 WOUND CARE MARKET: REGIONAL MIX, 2023-2030
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Growing geriatric population
- 5.2.1.2 Rise in cases of traumatic injuries
- 5.2.1.3 Growing prevalence of chronic diseases
- 5.2.1.4 Rising incidence of burn injuries
- 5.2.1.5 Rising use of regenerative medicines for wound management
- 5.2.1.6 Government initiatives and reimbursement policies
- 5.2.2 RESTRAINTS
- 5.2.2.1 High cost of advanced wound care products
- 5.2.2.2 Risks associated with advanced wound care products
- 5.2.2.3 Antimicrobial resistance challenges
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Growth opportunities in emerging economies
- 5.2.3.2 Technological advancements in wound care
- 5.2.3.3 Home care-optimized solutions
- 5.2.4 CHALLENGES
- 5.2.4.1 Lack of trained healthcare professionals
- 5.2.4.2 Limited access to wound care solutions in underdeveloped regions
- 5.2.4.3 Data security and privacy concerns
- 5.2.1 DRIVERS
- 5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 5.4 PRICING ANALYSIS
- 5.4.1 AVERAGE SELLING PRICE TREND, BY PRODUCT
- 5.4.2 AVERAGE SELLING PRICE TREND OF WOUND CARE PRODUCTS FOR KEY WOUND TYPES, BY KEY PLAYER
- 5.4.3 AVERAGE SELLING PRICE TREND, BY REGION
- 5.5 VALUE CHAIN ANALYSIS
- 5.6 SUPPLY CHAIN ANALYSIS
- 5.7 ECOSYSTEM ANALYSIS
- 5.8 INVESTMENT & FUNDING SCENARIO
- 5.9 TECHNOLOGY ANALYSIS
- 5.9.1 KEY TECHNOLOGIES
- 5.9.1.1 Negative pressure wound therapy
- 5.9.2 COMPLEMENTARY TECHNOLOGIES
- 5.9.2.1 Micropore particle technology
- 5.9.3 ADJACENT TECHNOLOGIES
- 5.9.3.1 Telemedicine and Mobile Health (MHealth)
- 5.9.1 KEY TECHNOLOGIES
- 5.10 PATENT ANALYSIS
- 5.11 TRADE ANALYSIS
- 5.11.1 IMPORT SCENARIO
- 5.11.2 EXPORT SCENARIO
- 5.12 KEY CONFERENCES AND EVENTS, 2025-2026
- 5.13 CASE STUDY ANALYSIS
- 5.13.1 CASE STUDY 1: ADOPTION OF ADVANCED WOUND CARE MEASURES FOR COMPLEX WOUND HEALING
- 5.13.2 CASE STUDY 2: EVALUATION OF NEW HYDROCOLL HYDROCOLLOID DRESSING IN WOUND CARE
- 5.13.3 CASE STUDY 3: SUPPORTED SELF-MANAGEMENT OF HARD-TO-HEAL PRESSURE ULCERS USING SIMPLE 3-STEP FRAMEWORK DEVELOPED BY COLOPLAST
- 5.14 REGULATORY LANDSCAPE
- 5.14.1 REGULATORY ANALYSIS
- 5.14.1.1 North America
- 5.14.1.1.1 US
- 5.14.1.1.2 Canada
- 5.14.1.2 Europe
- 5.14.1.2.1 Germany
- 5.14.1.2.2 UK
- 5.14.1.2.3 France
- 5.14.1.3 Asia Pacific
- 5.14.1.3.1 China
- 5.14.1.3.2 Japan
- 5.14.1.3.3 India
- 5.14.1.4 Latin America
- 5.14.1.5 Middle East
- 5.14.1.1 North America
- 5.14.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.14.2.1 North America
- 5.14.2.2 Europe
- 5.14.2.3 Asia Pacific
- 5.14.2.4 Latin America
- 5.14.2.5 Rest of the World
- 5.14.1 REGULATORY ANALYSIS
- 5.15 PORTER'S FIVE FORCES ANALYSIS
- 5.15.1 BARGAINING POWER OF SUPPLIERS
- 5.15.2 BARGAINING POWER OF BUYERS
- 5.15.3 THREAT FROM NEW ENTRANTS
- 5.15.4 THREAT FROM SUBSTITUTES
- 5.15.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.16 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.16.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.16.2 BUYING CRITERIA
- 5.17 IMPACT OF AI ON WOUND CARE MARKET
- 5.17.1 INTRODUCTION
- 5.17.2 MARKET POTENTIAL OF AI IN WOUND CARE MARKET
- 5.17.3 IMPACT OF AI ON WOUND CARE MARKET: USE CASES
- 5.17.4 KEY COMPANIES IMPLEMENTING AI
- 5.17.5 FUTURE OF AI IN WOUND CARE MARKET
- 5.18 US 2025 TARIFF
- 5.18.1 INTRODUCTION
- 5.18.2 KEY TARIFF RATES
- 5.18.3 PRICE IMPACT ANALYSIS
- 5.18.4 IMPACT ON COUNTRY/REGION
- 5.18.4.1 North America
- 5.18.4.2 Europe
- 5.18.4.3 Asia Pacific
- 5.18.5 IMPACT ON END-USE INDUSTRIES
- 5.18.5.1 Hospitals and clinics
6 WOUND CARE MARKET, BY PRODUCT
- 6.1 INTRODUCTION
- 6.2 ADVANCED WOUND CARE PRODUCTS
- 6.2.1 ADVANCED WOUND DRESSINGS, BY TYPE
- 6.2.1.1 Foam dressings
- 6.2.1.1.1 Silicone dressings
- 6.2.1.1.1.1 Ability of silicone dressings to expedite wound closure and reduce risk of maceration to drive market
- 6.2.1.1.2 Non-silicone dressings
- 6.2.1.1.2.1 Non-silicone dressings are designed for patient comfort and ease of use
- 6.2.1.1.1 Silicone dressings
- 6.2.1.2 Hydrocolloid dressings
- 6.2.1.2.1 Ability of hydrocolloid dressings to promote granulation and formation of new tissues in open wounds to boost demand
- 6.2.1.3 Film dressings
- 6.2.1.3.1 Film dressings offer strong adhesive properties and adaptability to various wound types
- 6.2.1.4 Alginate dressings
- 6.2.1.4.1 Growing incidence of pressure and diabetic foot ulcers to fuel growth
- 6.2.1.5 Hydrogel dressings
- 6.2.1.5.1 Ability of hydrogel dressings to provide relief and cooling effect on skin to drive market
- 6.2.1.6 Collagen dressings
- 6.2.1.6.1 Favorable reimbursement scenario for collagen dressings to support market
- 6.2.1.7 Hydrofiber dressings
- 6.2.1.7.1 Combination of properties of hydrocolloids and alginates to boost adoption of hydrofiber dressings
- 6.2.1.8 Wound contact layers
- 6.2.1.8.1 Ability of wound contact layers to protect wound beds from bacterial and fungal growth to drive market
- 6.2.1.9 Superabsorbent dressings
- 6.2.1.9.1 Use of superabsorbent dressings for fragile skin to promote growth
- 6.2.1.10 Other advanced wound care dressings
- 6.2.1.1 Foam dressings
- 6.2.2 ADVANCED WOUND DRESSINGS, BY PROPERTY
- 6.2.2.1 Non-antimicrobial dressings
- 6.2.2.1.1 Need for cost-effective dressing alternatives to drive market
- 6.2.2.2 Antimicrobial dressings
- 6.2.2.2.1 Advanced antimicrobial dressings are designed to prevent or manage infections in wounds
- 6.2.2.1 Non-antimicrobial dressings
- 6.2.3 DEVICES & ACCESSORIES
- 6.2.3.1 NPWT devices & accessories
- 6.2.3.1.1 Growing adoption of single-use NPWT devices in home care to drive growth
- 6.2.3.2 Debridement devices & accessories
- 6.2.3.2.1 Focus on developing novel technologies to support market
- 6.2.3.3 Wound assessment & monitoring devices
- 6.2.3.3.1 Potential cost-reducing capabilities of wound assessment & monitoring devices to boost adoption
- 6.2.3.4 Other devices & accessories
- 6.2.3.1 NPWT devices & accessories
- 6.2.4 BIOLOGICAL SKIN SUBSTITUTES
- 6.2.4.1 Human donor tissue-derived products
- 6.2.4.1.1 High efficacy of human donor tissue-derived products to support market demand
- 6.2.4.2 Acellular animal-derived products
- 6.2.4.2.1 Recognition of importance of ECM in wound treatment to lead to development of acellular wound care biologics
- 6.2.4.3 Biosynthetic products
- 6.2.4.3.1 Increased bioburden of infected wounds to lead to development of biosynthetic wound care products
- 6.2.4.1 Human donor tissue-derived products
- 6.2.5 TOPICAL AGENTS
- 6.2.5.1 Cost-effectiveness and ease of use of topical agents in home care settings to drive demand
- 6.2.1 ADVANCED WOUND DRESSINGS, BY TYPE
- 6.3 SURGICAL WOUND CARE PRODUCTS
- 6.3.1 SUTURES
- 6.3.1.1 High tensile strength of sutures to drive market
- 6.3.2 TISSUE ADHESIVES, SEALANTS, AND GLUES
- 6.3.2.1 Fibrin-based sealants
- 6.3.2.1.1 Fibrin-based sealants replicate final stages of body's natural blood-clotting process
- 6.3.2.2 Synthetic adhesives
- 6.3.2.2.1 Synthetic adhesives have additional sealing properties than traditional staples
- 6.3.2.3 Collagen-based sealants
- 6.3.2.3.1 Collagen-based sealants are primarily used in surgical procedures to support hemostasis
- 6.3.2.1 Fibrin-based sealants
- 6.3.3 STAPLERS
- 6.3.3.1 Staple lines are consistent and less likely to leak blood or air
- 6.3.1 SUTURES
- 6.4 TRADITIONAL WOUND CARE PRODUCTS
- 6.4.1 FIXATIONS
- 6.4.1.1 Fixation materials ensure dressings, wound pads, and compresses stay securely in place
- 6.4.2 BANDAGES
- 6.4.2.1 Wide usage of bandages to support market growth
- 6.4.3 GAUZES
- 6.4.3.1 Wide usage of gauzes in surgeries and wound packing to propel market growth
- 6.4.4 FIRST AID PLASTERS
- 6.4.4.1 Need for rapid wound management and protection to propel adoption
- 6.4.5 ABSORBENT PADS
- 6.4.5.1 Wide usage of absorbent pads in wound dressing and exudate management to drive market
- 6.4.6 COMPRESSIONS
- 6.4.6.1 Ability of compression products to reduce swelling to boost demand
- 6.4.7 ISLAND DRESSINGS
- 6.4.7.1 Waterproof, antimicrobial, and absorbent properties of island dressings to drive their demand
- 6.4.8 OTHER TRADITIONAL WOUND CARE PRODUCTS
- 6.4.1 FIXATIONS
7 WOUND CARE MARKET, BY WOUND TYPE
- 7.1 INTRODUCTION
- 7.2 CHRONIC WOUNDS
- 7.2.1 DIABETIC FOOT ULCERS
- 7.2.1.1 Rising incidence of diabetes to drive market growth
- 7.2.2 PRESSURE ULCERS
- 7.2.2.1 Growing geriatric population to drive market growth
- 7.2.3 VENOUS LEG ULCERS
- 7.2.3.1 Rising prevalence of obesity to drive market growth
- 7.2.4 OTHER CHRONIC WOUNDS
- 7.2.1 DIABETIC FOOT ULCERS
- 7.3 ACUTE WOUNDS
- 7.3.1 SURGICAL & TRAUMATIC WOUNDS
- 7.3.1.1 Rising volume of surgical procedures to drive market
- 7.3.2 BURNS
- 7.3.2.1 High incidence of burn injuries in developing regions to support market growth
- 7.3.1 SURGICAL & TRAUMATIC WOUNDS
8 WOUND CARE MARKET, BY END USER
- 8.1 INTRODUCTION
- 8.2 HOSPITALS & CLINICS
- 8.2.1 INPATIENT SETTINGS
- 8.2.1.1 Increasing hospital admissions due to HAIs to drive market
- 8.2.2 OUTPATIENT SETTINGS
- 8.2.2.1 Rising cost of inpatient facilities to spur market for outpatient care settings
- 8.2.1 INPATIENT SETTINGS
- 8.3 HOME CARE SETTINGS
- 8.3.1 DECREASED COSTS AND HIGH CONVENIENCE AND COMFORT OF HOME CARE SETTINGS TO ENSURE MARKET GROWTH
- 8.4 LONG-TERM CARE FACILITIES
- 8.4.1 GROWING GERIATRIC POPULATION TO BOOST NEED FOR BETTER LONG-TERM CARE
- 8.5 OTHER END USERS
9 WOUND CARE MARKET, BY REGION
- 9.1 INTRODUCTION
- 9.2 NORTH AMERICA
- 9.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA
- 9.2.2 US
- 9.2.2.1 Rising prevalence of diabetes and supportive reimbursement policies to drive growth
- 9.2.3 CANADA
- 9.2.3.1 Government-backed efforts to support market development
- 9.3 EUROPE
- 9.3.1 MACROECONOMIC OUTLOOK FOR EUROPE
- 9.3.2 GERMANY
- 9.3.2.1 Rising cases of diabetes and chronic wound burden to boost market
- 9.3.3 FRANCE
- 9.3.3.1 Rising chronic wound cases to drive market expansion
- 9.3.4 UK
- 9.3.4.1 Increase in R&D activity to propel market growth
- 9.3.5 ITALY
- 9.3.5.1 Increased healthcare funding and sustained demand for treatment solutions to drive market
- 9.3.6 SPAIN
- 9.3.6.1 Rising life expectancy and aging population to boost adoption of wound care products
- 9.3.7 RUSSIA
- 9.3.7.1 Government initiatives to raise awareness about diabetes to support market expansion
- 9.3.8 REST OF EUROPE
- 9.4 ASIA PACIFIC
- 9.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
- 9.4.2 CHINA
- 9.4.2.1 Increasing incidence of diabetic foot ulcers (DFUs) to encourage growth
- 9.4.3 JAPAN
- 9.4.3.1 Evolving healthcare delivery and infrastructure landscape to fuel market
- 9.4.4 INDIA
- 9.4.4.1 Booming medical tourism to speed up growth
- 9.4.5 AUSTRALIA
- 9.4.5.1 Growth of medical manufacturing companies to drive market
- 9.4.6 SOUTH KOREA
- 9.4.6.1 Increasing healthcare spending to sustain growth
- 9.4.7 SINGAPORE
- 9.4.7.1 Increasing focus on wound care therapies to facilitate growth
- 9.4.8 REST OF ASIA PACIFIC
- 9.5 LATIN AMERICA
- 9.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA
- 9.5.2 BRAZIL
- 9.5.2.1 Growth in healthcare sector to drive market
- 9.5.3 MEXICO
- 9.5.3.1 Expanding hospital infrastructure to aid growth
- 9.5.4 REST OF LATIN AMERICA
- 9.6 MIDDLE EAST & AFRICA
- 9.6.1 GROWING DEMAND FOR HEALTHCARE FACILITIES TO DRIVE MARKET
- 9.6.2 MACROECONOMIC OUTLOOK FOR MIDDLE EAST & AFRICA
10 COMPETITIVE LANDSCAPE
- 10.1 OVERVIEW
- 10.2 KEY PLAYER STRATEGIES/RIGHT TO WIN, 2024
- 10.2.1 OVERVIEW OF STRATEGIES ADOPTED BY PLAYERS IN WOUND CARE MARKET
- 10.3 REVENUE ANALYSIS, 2022-2024
- 10.4 MARKET SHARE ANALYSIS, 2024
- 10.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 10.5.1 STARS
- 10.5.2 EMERGING LEADERS
- 10.5.3 PERVASIVE PLAYERS
- 10.5.4 PARTICIPANTS
- 10.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 10.5.5.1 Company footprint
- 10.5.5.2 Region footprint
- 10.5.5.3 Product footprint
- 10.5.5.4 Wound type footprint
- 10.5.5.5 End user footprint
- 10.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 10.6.1 PROGRESSIVE COMPANIES
- 10.6.2 RESPONSIVE COMPANIES
- 10.6.3 DYNAMIC COMPANIES
- 10.6.4 STARTING BLOCKS
- 10.6.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 10.6.5.1 Detailed list of key startups/SMEs
- 10.6.5.2 Competitive benchmarking of key startups/SMEs
- 10.7 COMPANY VALUATION AND FINANCIAL METRICS
- 10.7.1 COMPANY VALUATION
- 10.7.2 FINANCIAL METRICS
- 10.8 BRAND/PRODUCT COMPARISON
- 10.9 COMPETITIVE SCENARIO
- 10.9.1 PRODUCT LAUNCHES & APPROVALS
- 10.9.2 DEALS
- 10.9.3 EXPANSIONS
- 10.9.4 OTHER DEVELOPMENTS
11 COMPANY PROFILES
- 11.1 KEY PLAYERS
- 11.1.1 SOLVENTUM
- 11.1.1.1 Business overview
- 11.1.1.2 Products offered
- 11.1.1.3 Recent developments
- 11.1.1.3.1 Product launches & approvals
- 11.1.1.3.2 Other developments
- 11.1.1.4 MnM view
- 11.1.1.4.1 Right to win
- 11.1.1.4.2 Strategic choices
- 11.1.1.4.3 Weaknesses & competitive threats
- 11.1.2 JOHNSON & JOHNSON SERVICES, INC. (ETHICON)
- 11.1.2.1 Business overview
- 11.1.2.2 Products offered
- 11.1.2.3 Recent developments
- 11.1.2.3.1 Product launches & approvals
- 11.1.2.4 MnM view
- 11.1.2.4.1 Right to win
- 11.1.2.4.2 Strategic choices
- 11.1.2.4.3 Weaknesses & competitive threats
- 11.1.3 SMITH+NEPHEW
- 11.1.3.1 Business overview
- 11.1.3.2 Products offered
- 11.1.3.3 Recent developments
- 11.1.3.3.1 Product launches & approvals
- 11.1.3.3.2 Deals
- 11.1.3.3.3 Expansions
- 11.1.3.3.4 Other developments
- 11.1.3.4 MnM view
- 11.1.3.4.1 Right to win
- 11.1.3.4.2 Strategic choices
- 11.1.3.4.3 Weaknesses & competitive threats
- 11.1.4 CARDINAL HEALTH
- 11.1.4.1 Business overview
- 11.1.4.2 Products offered
- 11.1.4.3 Recent developments
- 11.1.4.3.1 Deals
- 11.1.4.3.2 Expansions
- 11.1.4.4 MnM view
- 11.1.4.4.1 Right to win
- 11.1.4.4.2 Strategic choices
- 11.1.4.4.3 Weaknesses & competitive threats
- 11.1.5 MOLNLYCKE AB
- 11.1.5.1 Business overview
- 11.1.5.2 Products offered
- 11.1.5.3 Recent developments
- 11.1.5.3.1 Deals
- 11.1.5.3.2 Other developments
- 11.1.5.4 MnM view
- 11.1.5.4.1 Right to win
- 11.1.5.4.2 Strategic choices
- 11.1.5.4.3 Weaknesses & competitive threats
- 11.1.6 CONVATEC GROUP PLC
- 11.1.6.1 Business overview
- 11.1.6.2 Products offered
- 11.1.6.3 Recent developments
- 11.1.6.3.1 Product launches & approvals
- 11.1.6.3.2 Deals
- 11.1.7 COLOPLAST GROUP
- 11.1.7.1 Business overview
- 11.1.7.2 Products offered
- 11.1.7.3 Recent developments
- 11.1.7.3.1 Product launches & approvals
- 11.1.7.3.2 Deals
- 11.1.8 ORGANOGENESIS INC.
- 11.1.8.1 Business overview
- 11.1.8.2 Products offered
- 11.1.8.3 Recent developments
- 11.1.8.3.1 Product launches & approvals
- 11.1.8.3.2 Expansions
- 11.1.9 PAUL HARTMANN AG
- 11.1.9.1 Business overview
- 11.1.9.2 Products offered
- 11.1.9.3 Recent developments
- 11.1.9.3.1 Deals
- 11.1.10 MIMEDX GROUP, INC.
- 11.1.10.1 Business overview
- 11.1.10.2 Products offered
- 11.1.10.3 Recent developments
- 11.1.10.3.1 Product launches & approvals
- 11.1.11 OWENS & MINOR
- 11.1.11.1 Business overview
- 11.1.11.2 Products offered
- 11.1.11.3 Recent developments
- 11.1.11.3.1 Deals
- 11.1.11.3.2 Other developments
- 11.1.12 B. BRAUN SE
- 11.1.12.1 Business overview
- 11.1.12.2 Products offered
- 11.1.12.3 Recent developments
- 11.1.12.3.1 Expansions
- 11.1.12.3.2 Other developments
- 11.1.13 ESSITY AKTIEBOLAG
- 11.1.13.1 Business overview
- 11.1.13.2 Products offered
- 11.1.13.3 Recent developments
- 11.1.13.3.1 Other developments
- 11.1.14 INTEGRA LIFESCIENCES CORPORATION
- 11.1.14.1 Business overview
- 11.1.14.2 Products offered
- 11.1.14.3 Recent developments
- 11.1.14.3.1 Product launches & approvals
- 11.1.14.3.2 Other developments
- 11.1.15 AVERY DENNISON CORPORATION
- 11.1.15.1 Business overview
- 11.1.15.2 Products offered
- 11.1.15.3 Recent developments
- 11.1.15.3.1 Expansions
- 11.1.16 MATIV HOLDINGS, INC.
- 11.1.16.1 Business overview
- 11.1.16.2 Products offered
- 11.1.16.3 Recent developments
- 11.1.16.3.1 Deals
- 11.1.17 BIOVENTUS
- 11.1.17.1 Business overview
- 11.1.17.2 Products offered
- 11.1.17.3 Recent developments
- 11.1.17.3.1 Deals
- 11.1.17.3.2 Other developments
- 11.1.18 ZIMMER BIOMET
- 11.1.18.1 Business overview
- 11.1.18.2 Products offered
- 11.1.18.3 Recent developments
- 11.1.18.3.1 Deals
- 11.1.19 MEDTRONIC
- 11.1.19.1 Business overview
- 11.1.19.2 Products offered
- 11.1.20 BAXTER
- 11.1.20.1 Business overview
- 11.1.20.2 Products offered
- 11.1.20.3 Recent developments
- 11.1.20.3.1 Product launches & approvals
- 11.1.1 SOLVENTUM
- 11.2 OTHER PLAYERS
- 11.2.1 LOHMANN & RAUSCHER GMBH & CO. KG
- 11.2.2 MEDLINE INDUSTRIES, LP
- 11.2.3 DEROYAL INDUSTRIES, INC.
- 11.2.4 WINNER MEDICAL CO., LTD.
- 11.2.5 ADVANCIS (UK)
- 11.2.6 MIL LABORATORIES PVT. LTD.
- 11.2.7 PENSAR MEDICAL
- 11.2.8 HAROMED BV
- 11.2.9 URGO GROUP
- 11.2.10 DIRECT HEALTHCARE GROUP
12 APPENDIX
- 12.1 DISCUSSION GUIDE
- 12.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 12.3 CUSTOMIZATION OPTIONS
- 12.4 RELATED REPORTS
- 12.5 AUTHOR DETAILS



















