
|
시장보고서
상품코드
1771290
DNA 손상 반응 시장 : 업계 동향과 세계 예측 - 적응 질환별, 치료 영역별, 표적 분자별, 분자 유형별, 투여 경로별, 주요 지역별DNA Damage Response Market: Industry Trends and Global Forecasts - Distribution by Target Disease Indication, Therapeutic Area, Target Molecule, Type of Molecule, Route of Administration, and Key Geographical Regions |
||||||
세계 DNA 손상 반응 시장 : 개요
세계 DNA 손상 반응 시장 규모는 올해 1,300만 달러에 달했습니다. 이 시장은 예측 기간 동안 유리한 CAGR로 성장할 것으로 예측됩니다.
시장 세분화 및 기회 분석은 다음과 같은 매개 변수로 세분화됩니다.
적응증 질환
- 급성 골수성 백혈병
- COVID-19
- 당뇨병성 황반부종
- 중피종
- 골수 이형성 증후군
- 비편평 상피 비소세포 폐암
- 전립선암
- 자궁 장액암
치료 영역
- 혈액 악성 종양
- 고형암
- 기타
표적 분자
- APE1/Ref-1
- Casein Kinase 2
- CHK-1
- C-Tak
- DHODH
- MAPKAPK2
- p53
- WEE 1
분자 유형
- 생물학적 제제
- 저분자
투여 경로
- 경구약
- 정맥주사제
주요 지역
- 북미(미국, 캐나다)
- 유럽(덴마크, 프랑스, 독일, 이탈리아, 스페인, 영국)
- 아시아태평양(호주, 싱가포르, 한국)
세계 DNA 손상 반응 시장 : 성장과 동향
DNA 손상 반응(DDR)은 DNA 손상의 복구를 촉진할 뿐만 아니라 세포주기의 체크포인트를 활성화하는 경로의 협력적 네트워크로 구성되어 있습니다. 이는 전체 유전체의 무결성을 유지하기 위해 중요한 단계에서 세포주기의 정지로 이어져 전체 유전체의 무결성을 유지합니다. 특히 손상이 복구할 수 없는 경우, 이 복잡한 시스템은 세포가 분열하기 전에 유전 물질을 복구하거나 돌연변이 전파를 방지하기 위해 프로그램된 세포 사멸을 촉진하여 돌연변이 전파를 방지합니다. 또한, DDR은 기존 치료법에 대한 높은 특이성과 민감성, 낮은 표적 외 독성으로 인해 종양 및 비종양 질환을 포함한 광범위한 임상 증상에 대한 유망한 치료 표적이 되고 있습니다. 그 결과, 전 세계 연구자들은 DNA를 손상시키는 항암제 치료에 대한 DDR 매개 저항성에 대응하기 위해, 그리고 대체 경로를 표적으로 삼아 암에서 DDR 기능 장애를 활용하기 위해 DDR 억제제를 개발하고 있습니다.
DNA 손상 복구 과정을 표적으로 하는 4유형의 폴리 ADP 리보스 중합효소(PARP) 억제제가 현재 진행성 암 치료제로 승인되었습니다는 점은 주목할 만합니다. 또한, 전 세계 의약품 개발자들은 ATM, ATR, CHK1, WEE1 등 DNA 손상 반응 경로 내 다른 분자 표적들을 연구하고 있습니다.
세계 DNA 손상 대응 시장 : 주요 인사이트
이 보고서는 세계 DNA 손상 반응 시장의 현황을 조사하고 업계의 잠재적인 성장 기회를 파악합니다. 본 보고서의 주요 조사 결과는 다음과 같습니다.
- 현재 약 45개 기업이 다양한 임상 증상을 치료하기 위해 DNA 손상 반응(DDR)을 표적으로 하는 치료제를 개발하고 있습니다.
- 파이프라인에 있는 대부분의 약물 후보물질은 개발 초기 단계에 있으며, 주로 다양한 암 질환의 특징인 생체분자의 에피토프를 표적으로 삼도록 설계되어 있습니다.
- DDR을 표적으로 하는 치료제의 전임상 파이프라인은 풍부하고 성장하고 있습니다. 이들 약물 후보물질의 대부분(75% 이상)은 저분자 약물입니다.
- ATR을 표적으로 하는 약물 후보물질의 70% 이상이 임상시험 중이고, 약 55%는 경구 투여용으로 설계되어 있습니다.
- 고형암 치료를 위한 DDR 표적치료제의 약 65%는 이미 전임상시험을 통해 개념증명이 완료된 상태입니다.
- 2010년 이후 DDR을 표적으로 하는 치료제의 효능을 평가하기 위해 220개 이상의 임상시험이 등록되었지만, 아시아태평양에서는 이러한 임상시험의 평균 완료 기간이 상대적으로 짧은 것으로 나타났습니다.
- 이 분야에서의 혁신은 일류 저널에 게재된 수많은 과학 논문을 통해 확인할 수 있습니다. 현재 초점은 특히 다양한 유형의 암에 대한 새로운 표적 발굴에 맞추어져 있는 것으로 보입니다.
- 이 분야의 논문 수는 지난 1년간 크게 증가했으며, 논문의 약 30%는 2021년 이후에 발표된 논문입니다.
- 발표된 논문/기사에 따르면, 현재 연구 활동의 초점은 ATR, ADP, ATR, HSP 등의 표적 분자에 초점을 맞추었습니다.
- 백혈병, 폐암, 난소암 등 암에 대한 DDR 표적치료에 초점을 맞춘 연구가 눈에 띄게 증가하고 있습니다.
- 개발 초기 단계부터 약품 출시에 이르기까지 여러 가지 매개 변수가 가격 책정 및 채택률에 영향을 미칩니다. 개발자는 경쟁에서 승리하기 위해 이러한 모든 요인의 조합을 고려해야 합니다.
- 경쟁 우위를 추구하고 많은 소비자층을 확보하기 위해서는 혁신가들이 자사 제품의 채택과 가격 책정에 직간접적으로 영향을 미치는 요인을 이해하는 것이 필수적입니다.
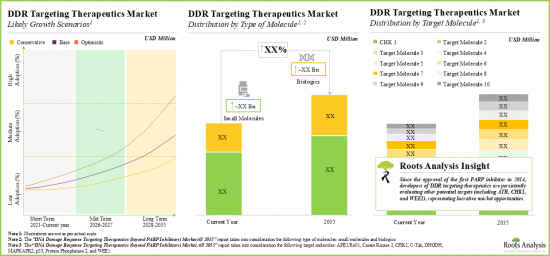
- 유망한 치료제 개발 파이프라인과 유망한 임상 연구 결과를 고려할 때, DDR 표적치료제 시장은 2035년까지 연평균 성장률이 크게 증가할 것으로 예측됩니다.
- 예상되는 시장 기회는 다양한 표적 질환 적응증, 투여 경로, 주요 지역에 걸쳐 충분히 분산되어 있을 가능성이 높습니다.
DNA 손상 대응 시장 진출기업 사례
- Aprea Therapeutics
- AstraZeneca
- Chordia Therapeutics
- Mission Therapeutics
- Repare Therapeutics
- Senhwa Biosciences
세계 DNA 손상 반응 시장
- 시장 규모 및 기회 분석 : 이 보고서는 세계 DNA 손상 반응 치료제 시장을 상세하게 분석하고,(A) 적응증 질환,(B) 치료 영역,(C) 표적 분자,(D) 분자 유형,(E) 투여 경로,(F) 주요 지역 등 주요 시장 부문에 초점을 맞추었습니다.
- 시장 상황:(A) 개발 단계,(B) 적응증 질환,(C) 치료 영역,(D) 표적 분자,(E) 분자 유형,(F) 치료 유형,(G) 제형,(H) 투여 경로,(I) 특효약 지정(있는 경우) 등 다양한 파라미터를 고려하여 DNA 손상 반응 표적 치료제를 종합적으로 평가합니다. 또한,(A) 설립연도,(B) 기업 규모(직원수),(C) 본사 소재지를 기준으로 DNA 손상반응 표적치료제 개발에 종사하는 기업을 종합적으로 평가합니다.
- 주요 인사이트【A】DNA 손상 반응 시장에 종사하는 주요 기업을 비교하는 버블 분석, 【B】DNA 손상 반응 표적 치료제 개발 기업을 표적 치료 영역과 기업 규모에 따라 분석, 【C】DNA 손상 반응 시장에 종사하는 개발 기업의 지역적 분포를 강조하는 상세 분석, 【D】DNA 손상 반응 표적 치료제의 분포도를 보여주는 종합 분석 등 4가지 도식을 통해 현대 시장 동향을 통찰력 있게 분석합니다.
- 기업 프로파일: A) 기업 개요,(B) 각 약물 후보물질 관련 세부 정보,(C) 최근 동향과 정보에 기반한 미래 전망에 초점을 맞춘 DNA 손상 반응 표적 치료제 개발 관련 기업의 상세한 프로파일.
- 임상시험 분석 : A) 임상시험 등록 연도, B) 등록 환자 수, C) 등록 환자 성별, D) 임상시험 단계, E) 모집 현황 및 시험 설계, F) 주요 스폰서/공동연구자 및 주요 진출기업(임상시험 수행 건수), G) 조직 유형, H) 인기 있는 치료법 지역,(i) 임상시험의 지역 분포 등 다양한 관련 파라미터를 기반으로 다양한 DNA 손상 반응 표적 치료제에 대한 250개 이상의 완료, 진행 및 계획 중인 임상시험을 상세하게 분석합니다.
- 논문 분석 :(A) 출판 연도,(B) 출판 유형,(C) 주요 연구 거점,(D) 가장 인기 있는 저자,(E) 수여된 보조금 제공,(F) 표적 분자,(G) 가장 인기 있는 저널을 기준으로 DNA 손상 반응 표적화 연구와 관련된 150개 이상의 피어 리뷰 과학 논문을 통찰력 있게 분석했습니다.
세계의 DNA 손상 반응 시장에 대해 조사했으며, 시장 개요와 함께 적응증별/치료 영역별/표적 분자별/분자 유형별/투여 경로별 동향, 지역별 동향, 시장 진출기업 프로파일 등을 정리하여 전해드립니다.
목차
제1장 서문
제2장 주요 요약
제3장 서론
- 본 장의 개요
- DNA 손상 개요
- DNA 손상 물질
- DNA 손상 반응 시스템
- DNA 수복 경로 유형
- 결론
제4장 시장 구도
- 본 장의 개요
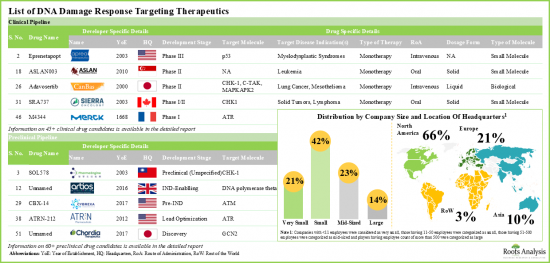
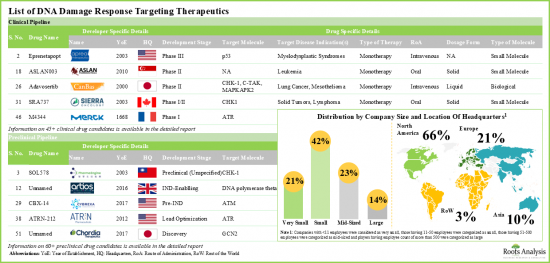
- DNA 손상 반응 표적 치료제 : 임상 파이프라인
- DNA 손상 반응 표적 치료제 : 전임상 파이프라인
- DNA 손상 반응 표적 치료제 : 개발자 리스트
제5장 주요 인사이트
- 본 장의 개요
- 포트폴리오의 힘, 개발 단계, 기업 규모별 분석(4D 버블 차트)
- 치료 영역과 기업 규모별 분석(트리 맵 표시)
- 본사 소재지별 분석(세계 지도 표시)
- 개발 단계, 치료 영역, 분자 유형, 치료법 유형, 투여 경로별 분석(그리드 표시)
제6장 기업 개요
- 본 장의 개요
- Aprea Therapeutics
- AstraZeneca
- Chordia Therapeutics
- Mission Therapeutics
- Repare Therapeutics
- Senhwa Biosciences
제7장 임상시험 분석
- 본 장의 개요
- 범위와 조사 방법
- DNA 손상 반응 표적 치료제 : 임상시험 분석
제8장 출판물 분석
- 본 장의 개요
- 범위와 조사 방법
- DNA 손상 반응 표적 치료제 : 최근 출판물 리스트
제9장 의약품 가격과 채택에 영향을 미치는 주요 파라미터 분석
- 본 장의 개요
- 주요 시장 성장 촉진요인
- 루트 분석 프레임워크
제10장 시장 예측
- 본 장의 개요
- 범위와 제한
- 예측 조사 방법과 주요 전제조건
- 2035년까지 세계의 DNA 손상 반응 표적 치료제 시장
- DNA 손상 반응 표적 치료제 시장 : 적응 질환별
- DNA 손상 반응 표적 치료제 시장 : 치료 영역별
- DNA 손상 반응 표적 치료제 시장 : 표적 분자별
- DNA 손상 반응 표적 치료제 시장 : 분자 유형별
- DNA 손상 반응 표적 치료제 시장 : 투여 경로별
- DNA 손상 반응 표적 치료제 시장 : 지역별
- 의약품별 판매 예측
- 결론
제11장 결론
제12장 부록 I : 표 형식 데이터
제13장 부록 II : 기업 및 조직 리스트
LSH 25.07.22GLOBAL DNA DAMAGE RESPONSE MARKET: OVERVIEW
As per Roots Analysis, the global DNA damage response market valued at USD 13 million in the current year is anticipated to grow at a lucrative CAGR during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Target Disease Indication
- Acute Myeloid Leukemias
- COVID-19
- Diabetic Macular Edemas
- Mesotheliomas
- Myelodysplastic Syndromes
- Non-Squamous Non-Small Cell Lung Cancers
- Prostate Cancers
- Uterine Serous Carcinomas
Therapeutic Area
- Hematological Malignancies
- Solid Tumors
- Other Disorders
Target Molecule
- APE1/Ref-1
- Casein Kinase 2
- CHK-1
- C-Tak
- DHODH
- MAPKAPK2
- p53
- WEE 1
Type of Molecule
- Biologics
- Small Molecule
Route of Administration
- Oral Drugs
- Intravenous Drugs
Key Geographical Regions
- North America (US and Canada)
- Europe (Denmark, France, Germany, Italy, Spain and UK)
- Asia-Pacific (Australia, Singapore and South Korea)
GLOBAL DNA DAMAGE RESPONSE MARKET: GROWTH AND TRENDS
The DNA damage response (DDR) consists of a coordinated network of pathways that not only facilitate the repair of DNA lesions but also activate cell cycle checkpoints. This leads to cell cycle arrest at critical stages in order to maintain the overall genomic integrity. Notably, if the damage is irreparable, this intricate system ensures that cells either repair their genetic material before undergoing division or facilitate programmed cell death to prevent the propagation of mutations. Further, its high specificity and sensitivity to conventional therapies, and low off-target toxicity have made DDR a promising therapeutic target for a broad range of clinical conditions, including both oncological and non-oncological diseases. Consequently, researchers worldwide are developing DDR inhibitors to counter DDR-mediated resistance to DNA-damaging anticancer therapies and to exploit DDR dysfunction in cancer by targeting alternative pathways.
It is worth mentioning that four poly-ADP ribose polymerase (PARP) inhibitor drugs, which target the DNA damage repair process, are currently approved for advanced-stage cancer treatment. Additionally, drug developers worldwide are investigating other molecular targets within the DNA damage response pathway, including ATM, ATR, CHK1, and WEE1.
GLOBAL DNA DAMAGE RESPONSE MARKET: KEY INSIGHTS
The report delves into the current state of global DNA damage response market and identifies potential growth opportunities within industry. Some key findings from the report include:
- Presently, close to 45 companies are engaged in the development of DNA damage response (DDR) targeting therapeutics in order to treat a range of clinical conditions.
- Majority of pipeline drug candidates are in the early phases of development; these are predominantly designed to target epitopes on biological molecules that are a characteristic of various oncological disorders.
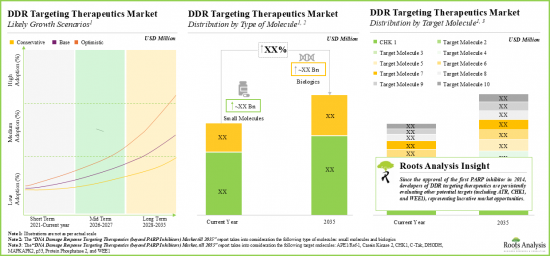
- The preclinical pipeline of DDR targeting therapeutics is substantial and growing; majority of these drug candidates (over 75%) are small molecules.
- Over 70% of drug candidates targeting ATR are in clinical trials and around 55% of such interventions are designed for oral administration.
- About 65% of DDR targeting drugs intended to treat solid tumors have already demonstrated preclinical proof of concept.
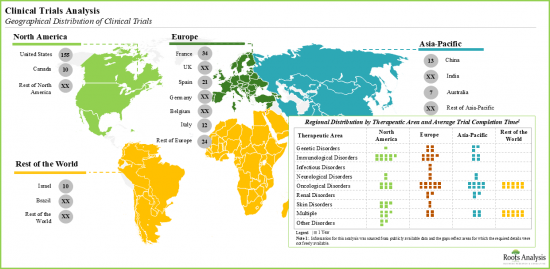
- Since 2010, over 220 clinical trials have been registered to evaluate the efficacy of DDR targeting therapies; average completion time for such studies was relatively less in Asia-Pacific.
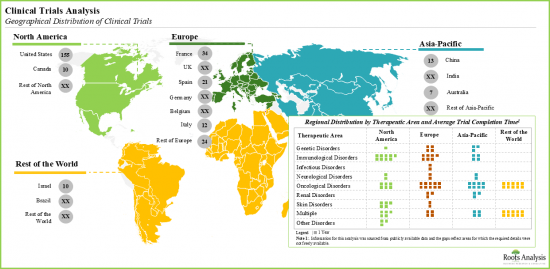
- Innovation in this field is evident across the plethora of scientific articles published in prestigious journals; the current focus appears to be on identification of novel targets, specifically against different types of cancers.
- The number of publications in this domain has increased significantly in the past one year; around 30% of the articles have been published since 2021.
- Published articles / papers indicate that the focus of current research activity is on target molecules, such as ATR, ADP, ATR and HSP.
- There has been an evident increase in research focused on DDR targeting therapies against cancers, such as leukemia, lung and ovarian cancers.
- Several parameters, ranging from initial stages of development to launch of the drug, influence the pricing and adoption rates; developers must consider the combination of all these factors to survive the competition.
- In pursuit of a competitive edge and the successful establishment of a large consumer base, it is imperative for innovators to understand both direct and indirect influences on the adoption and pricing of their respective products.
- Considering the promising development pipeline of therapies and encouraging clinical research outcomes, the DDR targeting therapeutics market is anticipated to grow at a significant annualized till 2035.
- The projected market opportunity is likely to be well-distributed across different target disease indications, routes of administration and key geographical regions.
Example Players in the DNA Damage Response Market
- Aprea Therapeutics
- AstraZeneca
- Chordia Therapeutics
- Mission Therapeutics
- Repare Therapeutics
- Senhwa Biosciences
GLOBAL DNA DAMAGE RESPONSE MARKET
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global DNA damage response market, focusing on key market segments, including [A] target disease indication, [B] therapeutic area, [C] target molecule, [D] type of molecule, [E] route of administration and [F] key geographical regions.
- Market Landscape: A comprehensive evaluation of DNA damage response targeting therapeutics, considering various parameters, such as [A] phase of development, [B] target disease indication(s), [C] therapeutic area, [D] target molecule, [E] type of molecule, [F] type of therapy, [G] dosage form, [H] route of administration and [I] special drug designation awarded (if any). Additionally, a comprehensive evaluation of the companies engaged in development of DNA damage response targeting therapeutics, based on [A] year of establishment, [B] company size (in terms of employee count) and [C] location of respective headquarters.
- Key Insights: An insightful analysis of contemporary market trends that have been depicted using four schematic representations, including [A] a bubble analysis comparing the leading players engaged in DNA damage response market, [B] an analysis of DNA damage response targeting therapeutics developers, based on their target therapeutic area and company size, [C] a detailed analysis highlighting the regional distribution of developers engaged in DNA damage response market, and [D] a comprehensive analysis illustrating the distribution of DNA damage response targeting therapeutics.
- Company Profiles: In-depth profiles of companies engaged in the development of DNA damage response targeting therapeutics, focusing on [A] company overview, [B] details related to its respective drug candidates and [C] recent developments and an informed future outlook.
- Clinical Trial Analysis: An in-depth analysis of more than 250 completed, ongoing and planned clinical studies of various DNA damage response targeting therapeutics, based on various relevant parameters, such as [A] trial registration year, [B] number of patients enrolled, [C] gender of patients enrolled, [D] trial phase, [E] recruitment status and study design, leading [F] sponsors / collaborators and leading players (in terms of number of trials conducted), [G] type of organization, [H] popular therapeutic areas and [I] regional distribution of trials.
- Publication Analysis: An insightful analysis of more than 150 peer-reviewed scientific articles related to DNA damage response targeting therapeutics, based on [A] year of publication, [B] type of publication, [C] key research hubs, [D] most popular authors, [E] provision of grant awarded, [F] target molecule, and [G] most popular journals.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Overview of DNA Damage
- 3.3. DNA Damaging Agents
- 3.4. DNA Damage Response Systems
- 3.4.1. Key Components of DNA Repair Pathways
- 3.5. Types of DNA Repair Pathways
- 3.5.1. Direct Pathways
- 3.5.2. Excision Repair Pathway
- 3.5.2.1. Base Excision Repair Pathway
- 3.5.2.2. Nucleotide Excision Repair Pathway
- 3.5.2.3. Mismatch Repair Pathway
- 3.5.3. Indirect Pathways
- 3.5.3.1. Homologous Recombination (HR) Repair Pathway
- 3.5.3.2. Non-homologous End Joining (NHEJ) Repair Pathway
- 3.6. Concluding Remarks
4. MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. DNA Damage Response Targeting Therapeutics: Clinical Pipeline
- 4.2.1. Analysis by Phase of Development
- 4.2.2. Analysis by Target Disease Indication(s)
- 4.2.3. Analysis by Therapeutic Area
- 4.2.4. Analysis by Target Molecule
- 4.2.5. Analysis by Type of Molecule
- 4.2.6. Analysis by Type of Therapy
- 4.2.7. Analysis by Dosage Form
- 4.2.8. Analysis by Route of Administration
- 4.2.9. Analysis by Special Drug Designation Awarded
- 4.3. DNA Damage Response Targeting Therapeutics: Preclinical Pipeline
- 4.3.1. Analysis by Phase of Development
- 4.3.2. Analysis by Target Disease Indication(s)
- 4.3.3. Analysis by Therapeutic Area
- 4.3.4. Analysis by Type of Molecule
- 4.3.5. Analysis by Type of Therapy
- 4.4 DNA Damage Response Targeting Therapeutics: List of Developers
- 4.4.1. Analysis by Year of Establishment
- 4.4.2. Analysis by Company Size
- 4.4.3. Analysis by Location of Headquarters
- 4.4.4. Leading Developers: Analysis by Number of Proprietary Product Candidates
5. KEY INSIGHTS
- 5.1. Chapter Overview
- 5.2. Analysis by Portfolio Strength, Phase of Development and Company Size (4D Bubble Chart)
- 5.3. Analysis by Therapeutic Area and Company Size (Treemap Representation)
- 5.4. Analysis by Location of Headquarters (World Map Representation)
- 5.5. Analysis by Phase of Development, Therapeutic Area, Type of Molecule, Type of Therapy and Route of Administration (Grid Representation)
6. COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. Aprea Therapeutics
- 6.2.1. Company Overview
- 6.2.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.2.3. Recent Developments and Future Outlook
- 6.3. AstraZeneca
- 6.3.1. Company Overview
- 6.3.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.3.3. Recent Developments and Future Outlook
- 6.4. Chordia Therapeutics
- 6.4.1. Company Overview
- 6.4.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.4.3. Recent Developments and Future Outlook
- 6.5. Mission Therapeutics
- 6.5.1. Company Overview
- 6.5.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.5.3. Recent Developments and Future Outlook
- 6.6. Repare Therapeutics
- 6.6.1. Company Overview
- 6.6.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.6.3. Recent Developments and Future Outlook
- 6.7. Senhwa Biosciences
- 6.7.1. Company Overview
- 6.7.2. DNA Damage Response Targeting Therapeutics Portfolio
- 6.7.3. Recent Developments and Future Outlook
7. CLINICAL TRIALS ANALYSIS
- 7.1. Chapter Overview
- 7.2. Scope and Methodology
- 7.3. DNA Damage Response Targeting Therapeutics: Clinical Trial Analysis
- 7.3.1. Analysis by Trial Registration Year
- 7.3.2. Analysis by Number of Patients Enrolled
- 7.3.3. Analysis by Gender of Patients Enrolled
- 7.3.4. Analysis by Trial Phase
- 7.3.5. Analysis by Recruitment Status
- 7.3.6. Analysis by Study Design
- 7.3.7. Analysis by Type of Sponsor / Collaborator
- 7.3.8. Analysis by Therapeutic Area
- 7.3.9. Reginal Analysis
- 7.3.10. Case Study
- 7.3.11. Most Active Industry Players: Analysis by Number of Clinical Trails
- 7.3.12. Concluding Remarks
8. PUBLICATION ANALYSIS
- 8.1. Chapter Overview
- 8.2. Scope and Methodology
- 8.3. DNA Damage Response Targeting Therapeutics: List of Recent Publications
- 8.3.1. Analysis by Year of Publication
- 8.3.2. Analysis by Type of Publication
- 8.3.3. Emerging Focus Areas
- 8.3.4. Analysis by Key Research Journals
- 8.3.4.1. Most Prominent Journals: Analysis by Number of Publications
- 8.3.4.2. Analysis by Journal Impact Factor
- 8.3.4.3. Most Prominent Journals: Analysis by Journal Impact Factor
- 8.3.5. Analysis by Key Research Hubs
- 8.3.6. Analysis by Target Molecule
- 8.3.6.1. Most Popular Target Molecule: Analysis by Number of Publications
- 8.3.6.2. Analysis by Year and Target Molecule
- 8.3.7. Analysis by Grants Awarded
- 8.3.7.1. Locations of Grant Awarding Organizations: Analysis by Number of Publications
- 8.3.8. Publication Benchmarking Analysis
9. ANALYSIS OF KEY PARAMETERS IMPACTING DRUG PRICING AND ADOPTION
- 9.1. Chapter Overview
- 9.2. Key Market Drivers
- 9.3. Roots Analysis Framework
- 9.3.1. Benchmarking Parameters
- 9.3.2. Methodology
- 9.3.3. Impact on Price and Adoption
- 9.3.4. Impact on Pricing and Adoption of Individual Drugs / Drug Candidates
- 9.3.4.1. Adavosertib
- 9.3.4.2. APX3330
- 9.3.4.3. ASLAN003
- 9.3.4.4. CBP-501
- 9.3.4.5. Eprenetapopt
- 9.3.4.6. Irofulven
- 9.3.4.7. LB-100
- 9.3.4.8. Silmitasertib
- 9.3.4.9. TRC-102
- 9.3.5. Concluding Remarks
10. MARKET FORECAST
- 10.1. Chapter Overview
- 10.2. Scope and Limitations
- 10.3. Forecast Methodology and Key Assumptions
- 10.4. Global DNA Damage Response Targeting Therapeutics Market, Till 2035
- 10.4.1. DNA Damage Response Targeting Therapeutics Market: Distribution by Target Disease Indication
- 10.4.1.1. DNA Damage Response Targeting Therapeutics Market for Acute Myeloid Leukemias, Till 2035
- 10.4.1.2. DNA Damage Response Targeting Therapeutics Market for COVID-19, Till 2035
- 10.4.1.3. DNA Damage Response Targeting Therapeutics Market for Diabetic Macular Edemas, Till 2035
- 10.4.1.4. DNA Damage Response Targeting Therapeutics Market for Mesotheliomas, Till 2035
- 10.4.1.5. DNA Damage Response Targeting Therapeutics Market for Myelodysplastic Syndromes, Till 2035
- 10.4.1.6. DNA Damage Response Targeting Therapeutics Market for Non-Squamous Non-Small Cell Lung Cancers, Till 2035
- 10.4.1.7. DNA Damage Response Targeting Therapeutics Market for Prostate Cancers, Till 2035
- 10.4.1.8. DNA Damage Response Targeting Therapeutics Market for Uterine Serous Carcinomas, Till 2035
- 10.4.2. DNA Damage Response Targeting Therapeutics Market: Distribution by Therapeutic Area
- 10.4.2.1. DNA Damage Response Targeting Therapeutics Market for Hematological Malignancies, Till 2035
- 10.4.2.2. DNA Damage Response Targeting Therapeutics Market for Solid Tumors, Till 2035
- 10.4.2.3. DNA Damage Response Targeting Therapeutics Market for Other Disorders, Till 2035
- 10.4.3. DNA Damage Response Targeting Therapeutics Market: Distribution by Target Molecule
- 10.4.3.1. DNA Damage Response Targeting Therapeutics Market for APE1/Ref-1, Till 2035
- 10.4.3.2. DNA Damage Response Targeting Therapeutics Market for Casein Kinase 2, Till 2035
- 10.4.3.3. DNA Damage Response Targeting Therapeutics Market for CHK-1, Till 2035
- 10.4.3.4. DNA Damage Response Targeting Therapeutics Market for C-Tak, Till 2035
- 10.4.3.5. DNA Damage Response Targeting Therapeutics Market for DHODH, Till 2035
- 10.4.3.6. DNA Damage Response Targeting Therapeutics Market for MAPKAPK2, Till 2035
- 10.4.3.7. DNA Damage Response Targeting Therapeutics Market for p53, Till 2035
- 10.4.3.8. DNA Damage Response Targeting Therapeutics Market for Protein Phosphatase 2A, Till 2035
- 10.4.3.9. DNA Damage Response Targeting Therapeutics Market for WEE1, Till 2035
- 10.4.4. DNA Damage Response Targeting Therapeutics Market: Distribution by Type of Molecule
- 10.4.4.1. DNA Damage Response Targeting Therapeutics Market for Biologics, Till 2035
- 10.4.4.2. DNA Damage Response Targeting Therapeutics Market for Small Molecules, Till 2035
- 10.4.5. DNA Damage Response Targeting Therapeutics Market: Distribution by Route of Administration
- 10.4.5.1. DNA Damage Response Targeting Therapeutics Market for Oral Drugs, Till 2035
- 10.4.5.2. DNA Damage Response Targeting Therapeutics Market for Intravenous Drugs, Till 2035
- 10.4.6. DNA Damage Response Targeting Therapeutics Market: Distribution by Geography
- 10.4.6.1. DNA Damage Response Targeting Therapeutics Market in the US, Till 2035
- 10.4.6.2. DNA Damage Response Targeting Therapeutics Market in Canada, Till 2035
- 10.4.6.3. DNA Damage Response Targeting Therapeutics Market in Denmark, Till 2035
- 10.4.6.4. DNA Damage Response Targeting Therapeutics Market in France, Till 2035
- 10.4.6.5. DNA Damage Response Targeting Therapeutics Market in Germany, Till 2035
- 10.4.6.6. DNA Damage Response Targeting Therapeutics Market in Italy, Till 2035
- 10.4.6.7. DNA Damage Response Targeting Therapeutics Market in Spain, Till 2035
- 10.4.6.8. DNA Damage Response Targeting Therapeutics Market in the UK, Till 2035
- 10.4.6.9. DNA Damage Response Targeting Therapeutics Market in Australia, Till 2035
- 10.4.6.10. DNA Damage Response Targeting Therapeutics Market in Singapore, Till 2035
- 10.4.6.11. DNA Damage Response Targeting Therapeutics Market in South Korea, Till 2035
- 10.4.7. Drug-wise Sales Forecast
- 10.4.7.1 Adavosertib (AZD1775, MK-1775), AstraZeneca
- 10.4.7.1.1. Target Patient Population
- 10.4.7.1.2. Sales Forecast
- 10.4.7.1.3. Net Present Value
- 10.4.7.1.4. Value Creation Analysis
- 10.4.7.2. APX3330, Apexian Pharmaceuticals
- 10.4.7.2.1. Target Patient Population
- 10.4.7.2.2. Sales Forecast
- 10.4.7.2.3. Net Present Value
- 10.4.7.2.4. Value Creation Analysis
- 10.4.7.3. ASLAN003 (LAS 186323), Aslan Pharmaceuticals
- 10.4.7.3.1. Target Patient Population
- 10.4.7.3.2. Sales Forecast
- 10.4.7.3.3. Net Present Value
- 10.4.7.3.4. Value Creation Analysis
- 10.4.7.4. CBP-501, CanBas
- 10.4.7.4.1. Target Patient Population
- 10.4.7.4.2. Sales Forecast
- 10.4.7.4.3. Net Present Value
- 10.4.7.4.4. Value Creation Analysis
- 10.4.7.5. Eprenetapopt, Aprea Therapeutics
- 10.4.7.5.1. Target Patient Population
- 10.4.7.5.2. Sales Forecast
- 10.4.7.5.3. Net Present Value
- 10.4.7.5.4. Value Creation Analysis
- 10.4.7.6. Irofulven, Allarity Therapeutics
- 10.4.7.6.1. Target Patient Population
- 10.4.7.6.2. Sales Forecast
- 10.4.7.6.3. Net Present Value
- 10.4.7.6.4. Value Creation Analysis
- 10.4.7.7. LB-100 (Lixte Biotechnology)
- 10.4.7.7.1. Target Patient Population
- 10.4.7.7.2. Sales Forecast
- 10.4.7.7.3. Net Present Value
- 10.4.7.7.4. Value Creation Analysis
- 10.4.7.8. Silmitasertib, Senhwa Biosciences
- 10.4.7.8.1. Target Patient Population
- 10.4.7.8.2. Sales Forecast
- 10.4.7.8.3. Net Present Value
- 10.4.7.8.4. Value Creation Analysis
- 10.4.7.1 Adavosertib (AZD1775, MK-1775), AstraZeneca
- 10.4.9 Concluding Remarks
- 10.4.1. DNA Damage Response Targeting Therapeutics Market: Distribution by Target Disease Indication



















